LiveDrop SA, Karolinska Institute, SciLifeLab & UPM Biomedicals
ORGANOID
The effectiveness of live cell shipment in the large scale production of liver spheroids for drug screening
April 2025
Spheroids have emerged as indispensable tools research and industry, offering improved physiological relevance, accelerated drug screening, and enhanced toxicity assessment compared to traditional 2D cultures.
However, hanging drop microplates can be labor-intensive, expensive, and limit scalability for high-throughput screening. Moreover, traditional large spheroids pose significant challenges for reproducible analysis using microscopy imaging techniques.
To overcome these challenges, we have developed a stream-lined workflow combining the high-throughput (HTP) miniaturized spheroid production capabilities of the OneFlow™ or ModaFlow™ LiveDrop’s microfluidics instruments with the benefits of GrowDex® hydrogel from UPM Biomedicals UPM medicals and live cell shipping technology of Cellbox Solutions.

The Project
This collaborative effort introduces an innovative approach to efficiently produce miniaturized spheroids, yielding 20,000 spheroids from 0.8 million cells suspended in 100 μL medium in just 3 minutes, with each spheroid measuring approximately 70 μm in diameter.
These spheroids exhibit robustness, homogeneity, and functionality. The incubation of cells in nanoliter-scale droplets promotes cell contact, facilitating cell aggregation and accelerating spheroid formation.
Additionally, the use of GrowDex® hydrogel provides steric hindrance to spheroids, preventing clustering during drug testing and cell staining. Furthermore, shipping live spheroids in a Cellbox™ portable CO₂ incubator ensures that optimal conditions for cell health and performance are maintained during transport.
This workflow reliability and simplicity unlocks the potential for further development to accommodate various cell types, expanding the potential for novel therapeutics and personalized medicine applications.
The Procedure
Overview of the drug screening workflow.

The Results
A

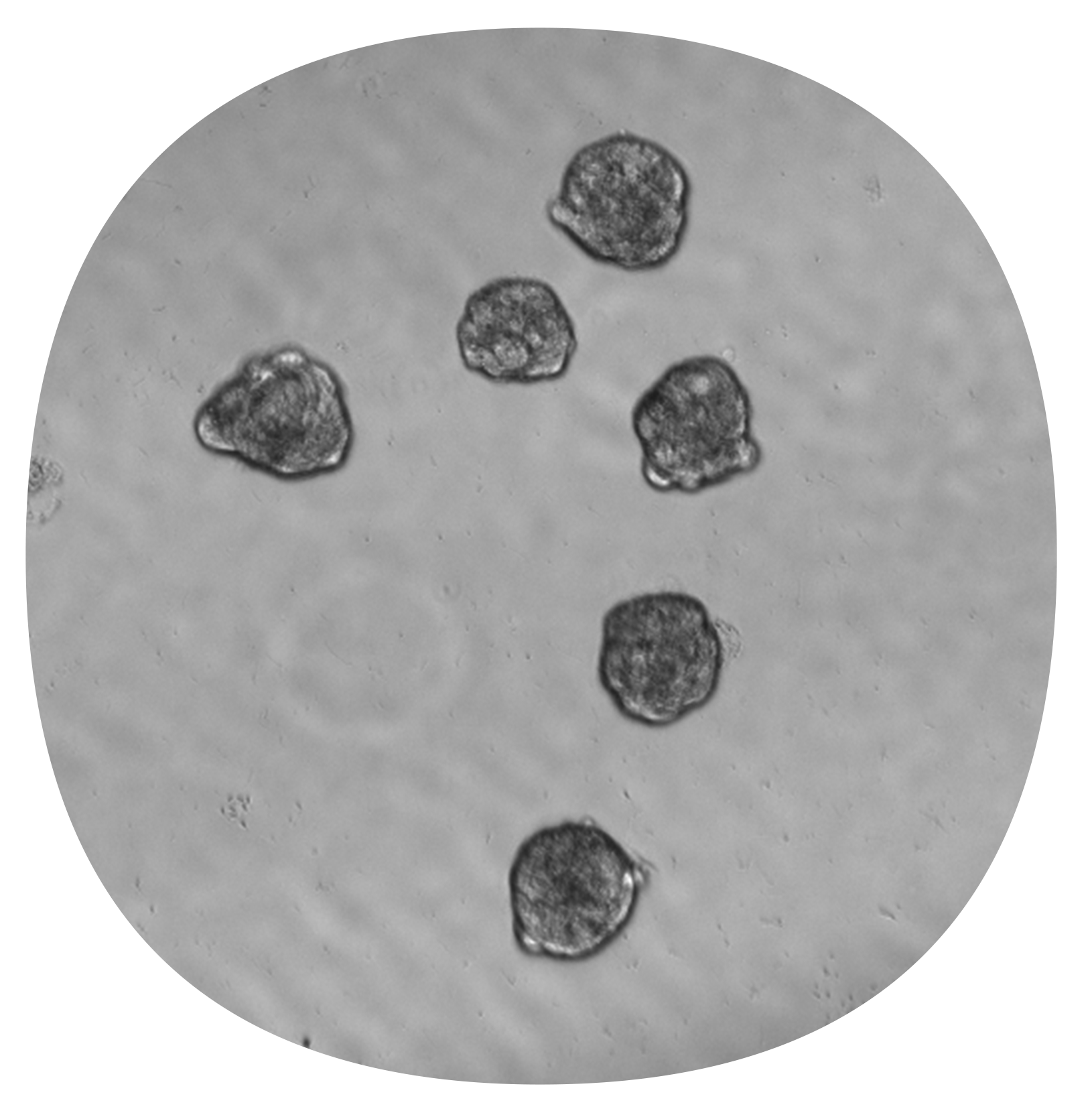
After 48-hour incubation in GrowDex® hydrogel which included a transport from Helsinki to Solna (A), the average diameter of spheroids was 70 μm (B) and the average roundness was 0.72 out of 1 (C).
After Cellbox transport in 384W plates maintained at 37C and 5% CO2 from Helsinki to Solna during a 48-hour incubation in GrowDex® hydrogel (A), the average diameter of spheroids was 70 μm (B) and the average roundness was 0.72 out of 1 (C).
B
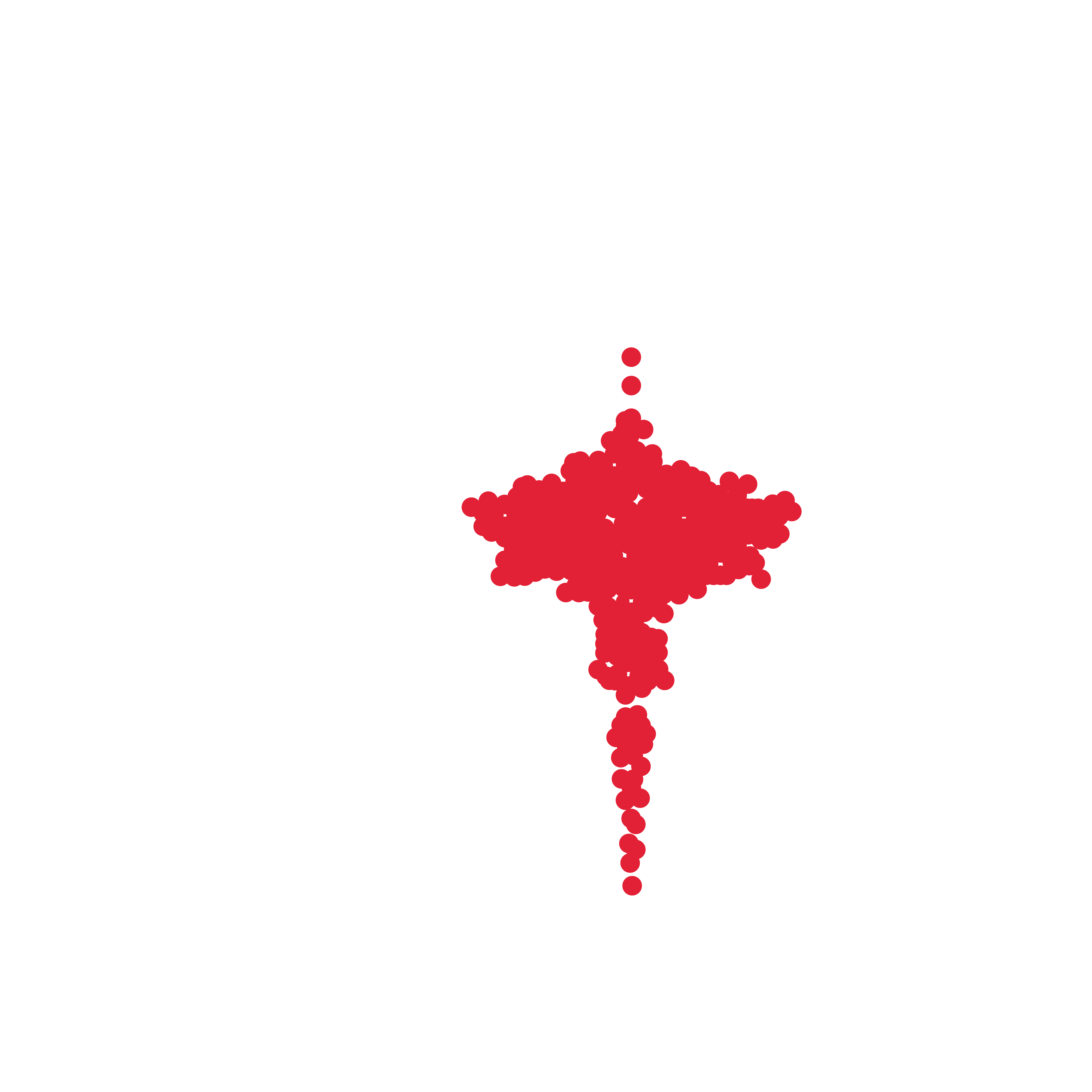
Diameter of released spheroids after overnight incubation. (μm)
C
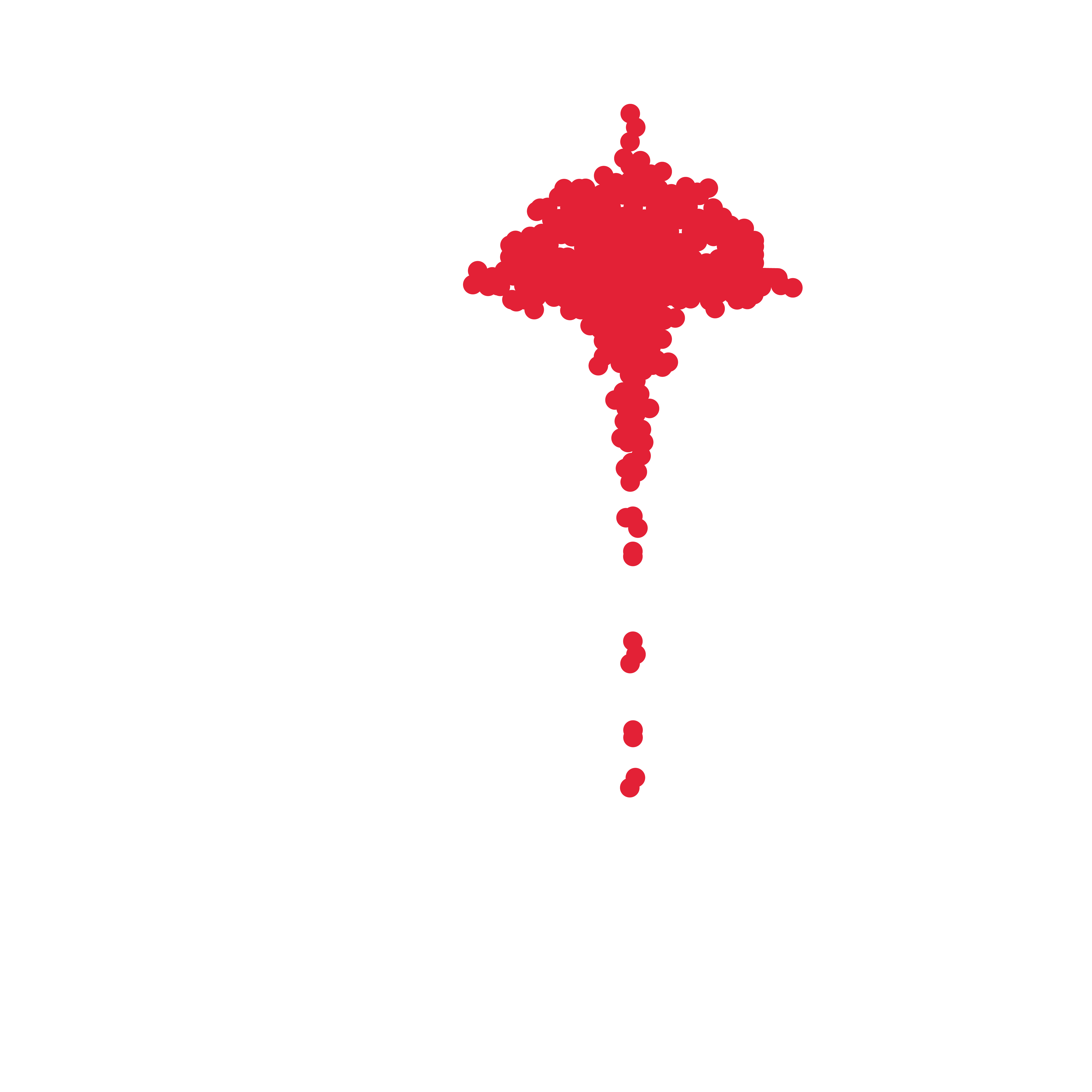
Roundness of released spheroids after overnight incubation (0-1)
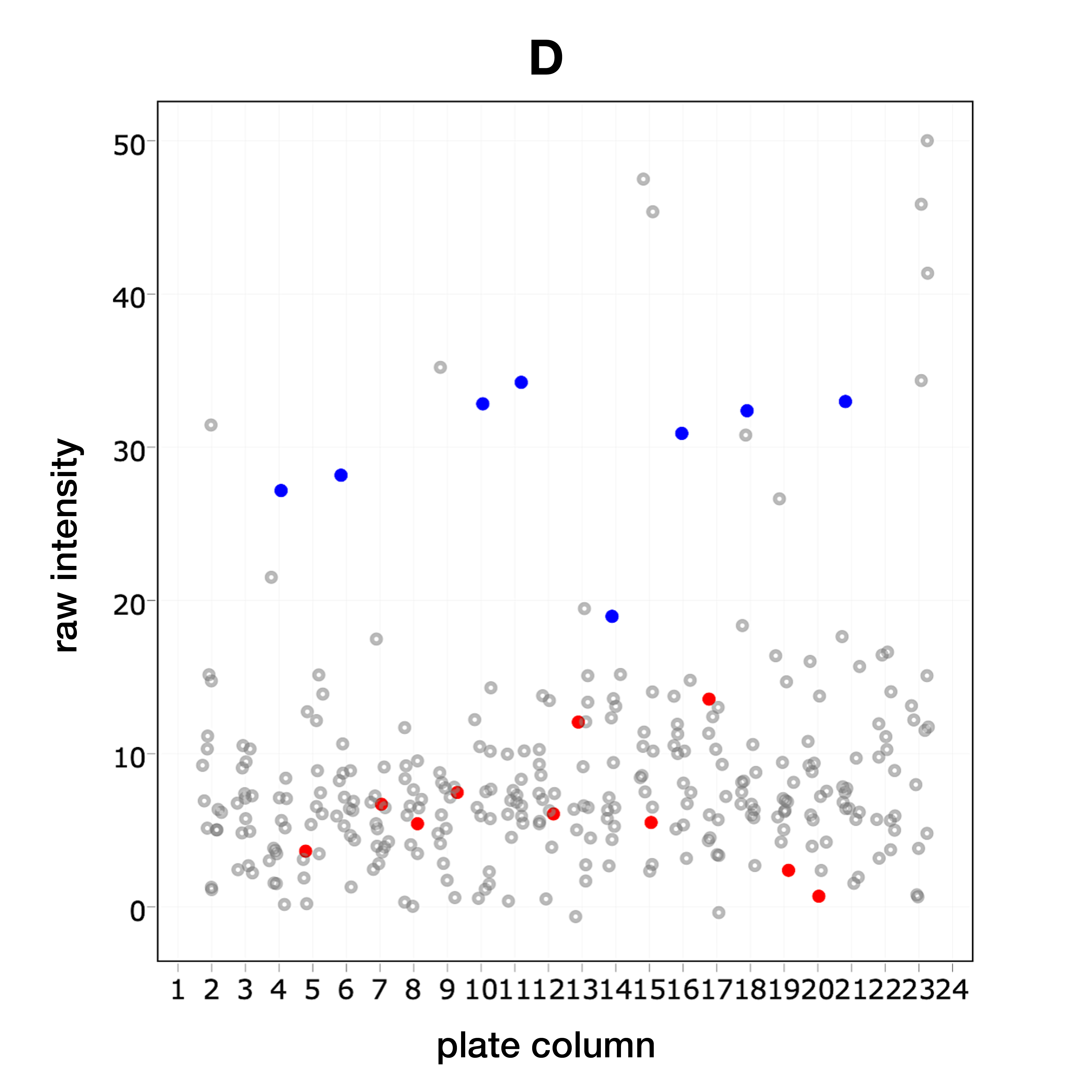
During HTS, the negative (DMSO) and positive (Benzoyl chloride) controls were distinguished (D) by measuring the cell viability by TMRM (orange, staining for live cells)-SyTox (green, staining for dead cells) staining.
• neg
• pos
• samples
E
F

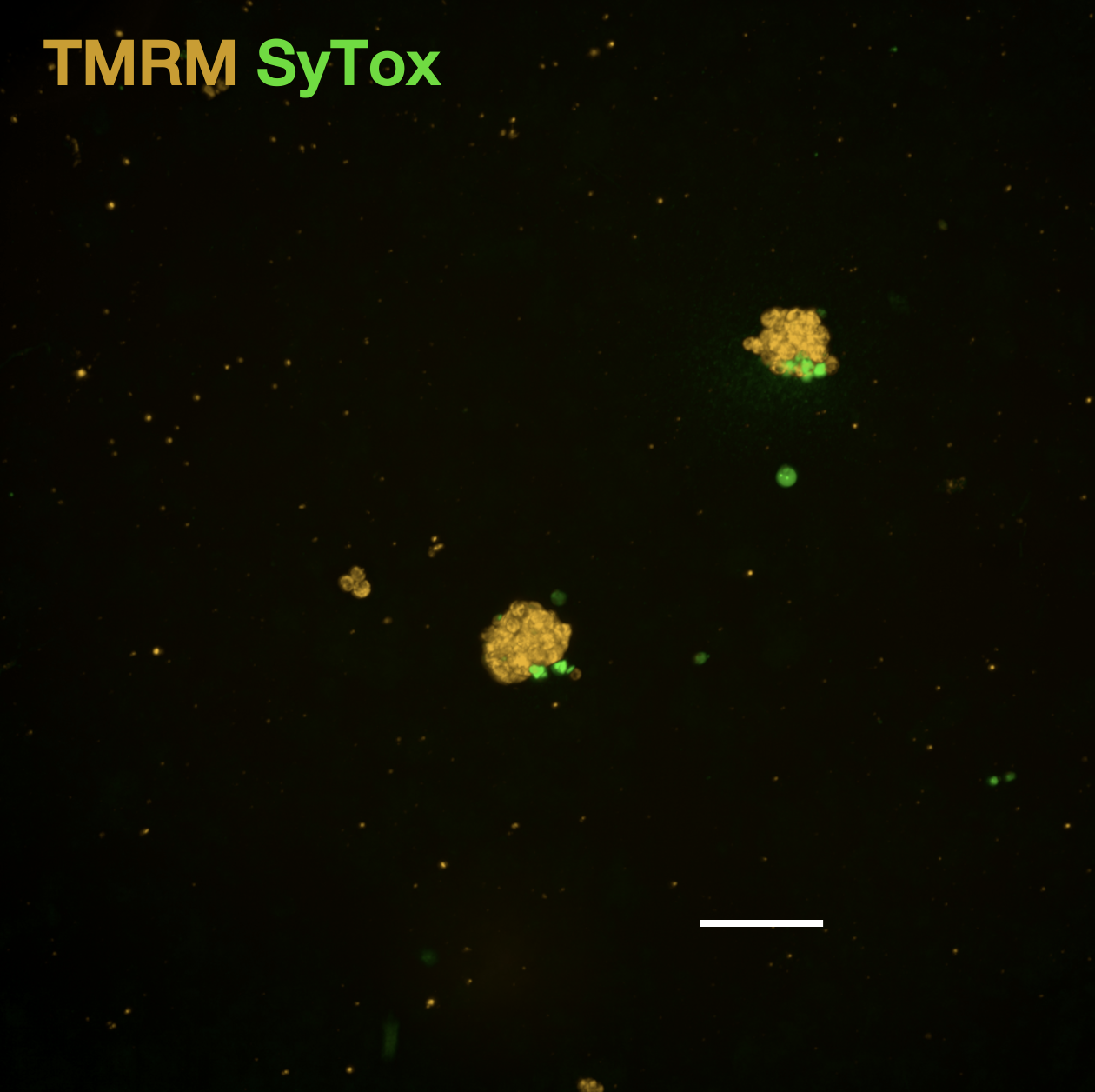
Cell viability of native control wells averaged 94% with example TMRM-SyTox staining of negative (E) and positive (F) control-treated spheroids (scale bars = 200 μm).
Karolinska Institute and SciLifeLab, Sweden:
Heshuang Qu, Salomé Thüler & Brinton Seashore-Ludlow
LiveDrop SA , Belgium:
Yacine Bounab, Stéphanie van Loo & Quentin Graillet
UPM Biomedicals, Finland: Piia Mikkonen

