UPM Biomedicals & Max Plank institute for molecular Biomedicine in Münster

ORGANOID
On the road again: Human Midbrain Organoids
Corné Swart, Katharina Becker, Martha Grabos, Theresa Kagermeier, Tony Kiuru, Lauri Paasonen, Henrik Renner, Jonathan Shead, Robin Sieg, Hans Schöler, Jan Bruder
January 2020
The Abstract
Drug discovery is a lengthy and expensive process, which in the majority of cases fail to deliver a safe and viable treatment for patients. In an attempt to improve the success rate of drug development, scientists and physicians are devising novel and often complex biological tools that mimic in vivo conditions in a more reliable manner than the current 2D cell cultures or animal models. The increased complexity of these in vivo-like biological structures exemplified in 3D cell cultures and engineered tissues present a challenge for the standard cryo-transport procedures. In the standard approach, cells are exposed to at least one freeze-thaw cycle which is known to cause cell death, reduction in cell proliferative capacity and altered gene expression.

Furthermore, cells are affected by the metabolic activity and toxicity of the cryoprotectancts that are integral to the cryopreservation process. To complicate matters even more, many sensitive cells and tissues are partly or completely incompatible with cryopreservation, limiting their application in high-throughput or high-content screening workflows where transport between facilities is unavoidable.
To investigate the effects of transport on fragile 3D cell cultures, we have selected human midbrain organoids as a model system. This study represents the first known attempt to transport midbrain organoids under laboratory incubation conditions (37 °C and 5 % CO₂). The organoids were packaged according to UN3373 guidelines and transported for more than 8 hours by road in an autarkic environmental device, the Cellbox™ Shipper.
In addition to the effects of transport in liquid media, we also investigated the effects of immobilizing the organoids in GrowDex® wood nanocellulose based hydrogels manufactured by UPM Biomedicals. We anticipated that this would serve as a protective buffer against shock, vibration and possible sample loss. Our study found that transported human midbrain organoids experienced no changes in their viability as a result of being transported in the Cellbox™. This study therefore validates the device as a feasible solution for the transport of organoids.
By comparing organoids which have been grown in culture media with and without the addition of GrowDex® or GrowDex®-T, we could also show that GrowDex® hydrogels significantly increases cell viability, not only during transport but also during stationary incubation, suggesting a protective role in the culturing of these organoids.

Experimental Aims
- Validation of a commercially available transportable incubator for the safe transport of midbrain organoids under laboratory conditions.
- lnvestigate the influence of a natural nanocellulose based hydrogel during stationary incubation and transport of midbrain organoids.
Hydrogel as ECM
Human stem cell based neuronal cultures are a promising tool for studying disease mechanisms, drug response or developmental biology in vitro. 2D culture can lead to unnatural cell polarization on the flat culture. Different types of 3D cell cultures aim to overcome some of these limitations, by offering cells an extracellular matrix (ECM) to better mimic the in vivo environment.
GrowDex® hydrogels are a range of nanofibrillar cellulose based hydrogels which are ready to use, mimic the ECM, whilst supporting cell growth and differentiation with consistent results. Previously, pre-differentiated human pluripotent stem cell derived neurons where successfully encapsulated and cultured within GrowDex® (App Note 15).
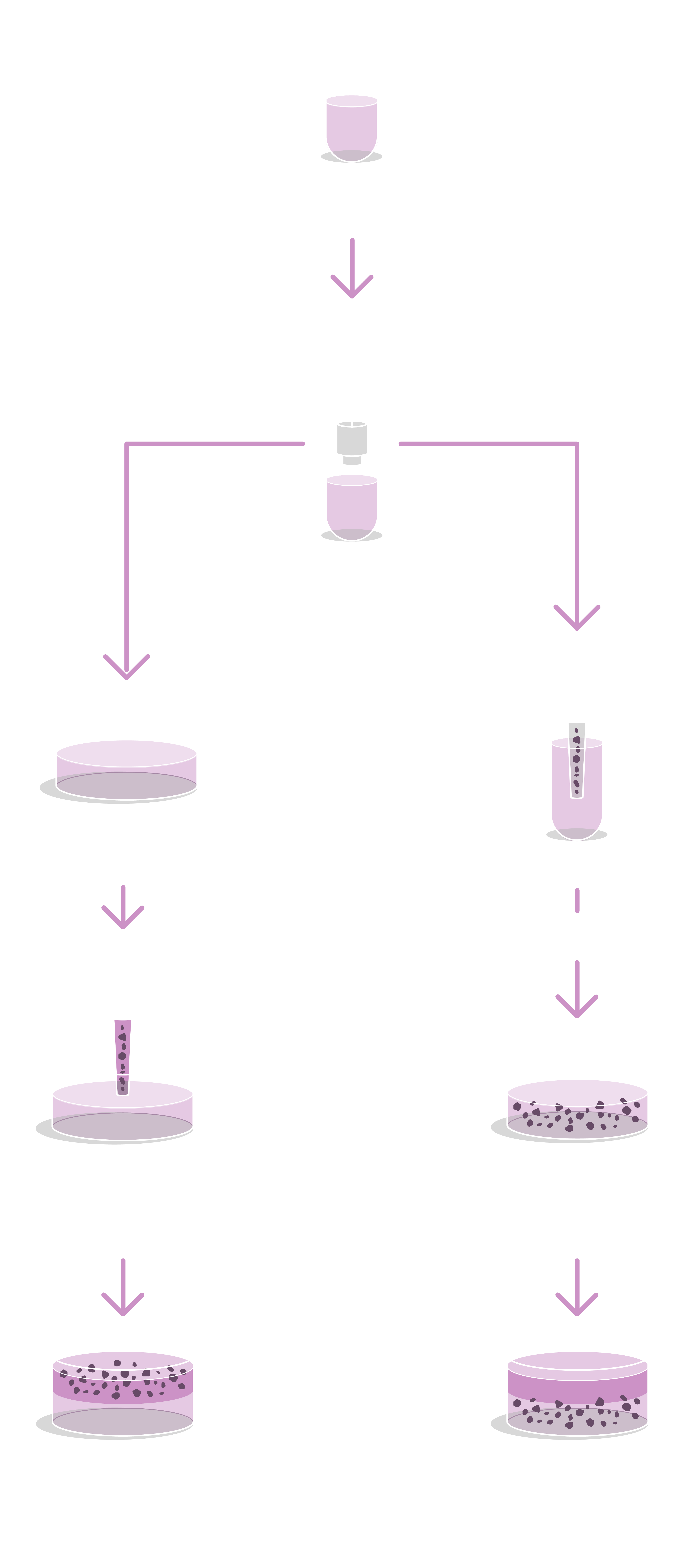
GrowDex® products are ideal for these applications because they are animal-free, room temperature stable, ready-to-use, reproducible, biocompatible, microscope and image compatible and have tunable viscosity. Handling protocols for GrowDex® are illustrated below.

Assay Set Up
Cargo & Packaging
Midbrain organoids are routinely cultivated in the laboratory in standard incubators at 37° C and 5 % CO₂. Deviations from these conditions will have a negative impact on the cells and may lead to sample variation and cell death.
lncompatibility with cryo-transport procedures demand innovative solutions like the Cellbox™ shipping incubator which can be used to transport midbrain and other organoids under laboratory conditions.

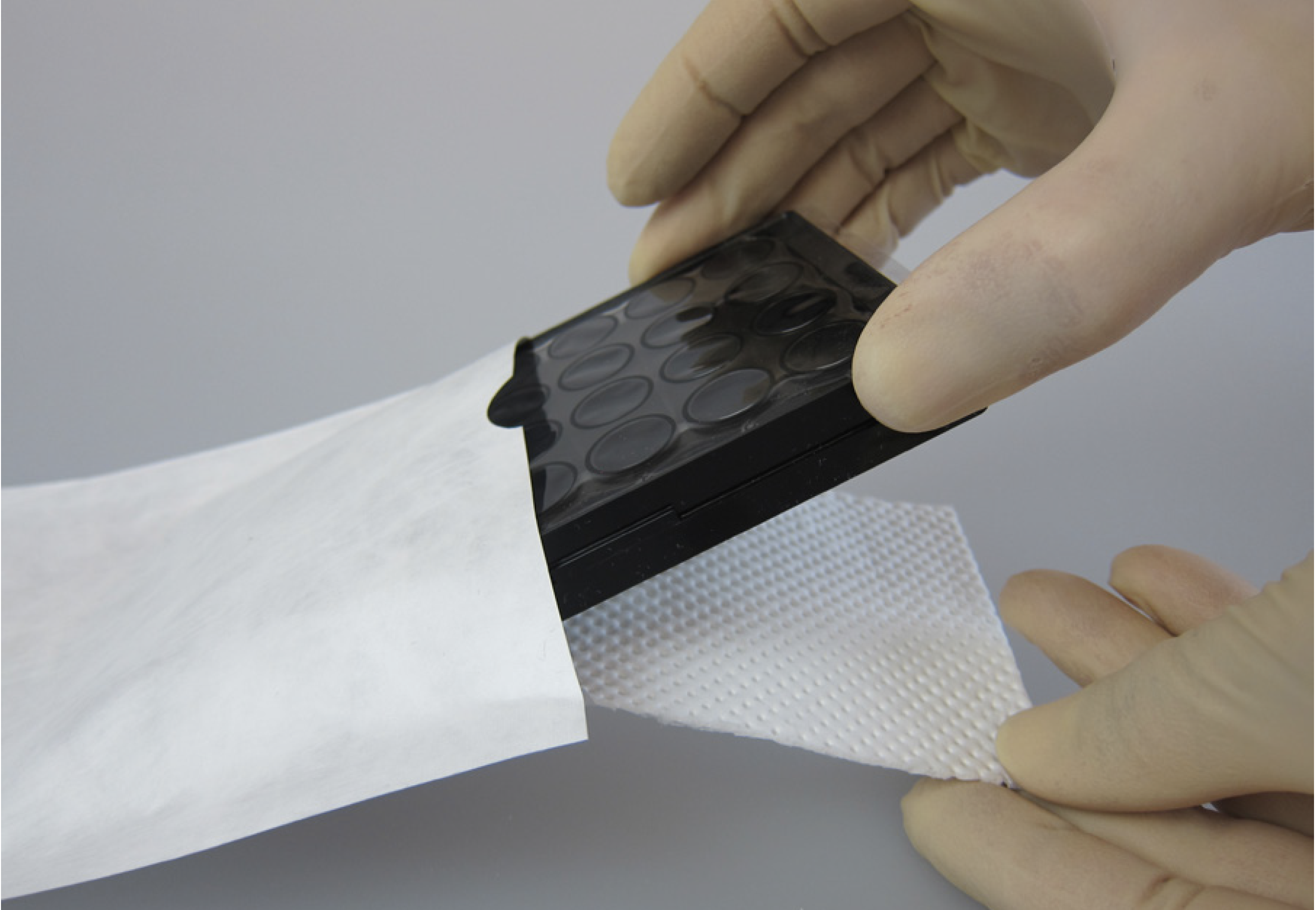
- Fulfills UN 3373
- Threefold package: gas permeable adhesive film, Tyvek-bag + absorber material, Cellbox™ Lid
- Suitable package solution for all established culture vessels such as multiwell-plates, T-flasks and chips
Data Logging & Transport Validation
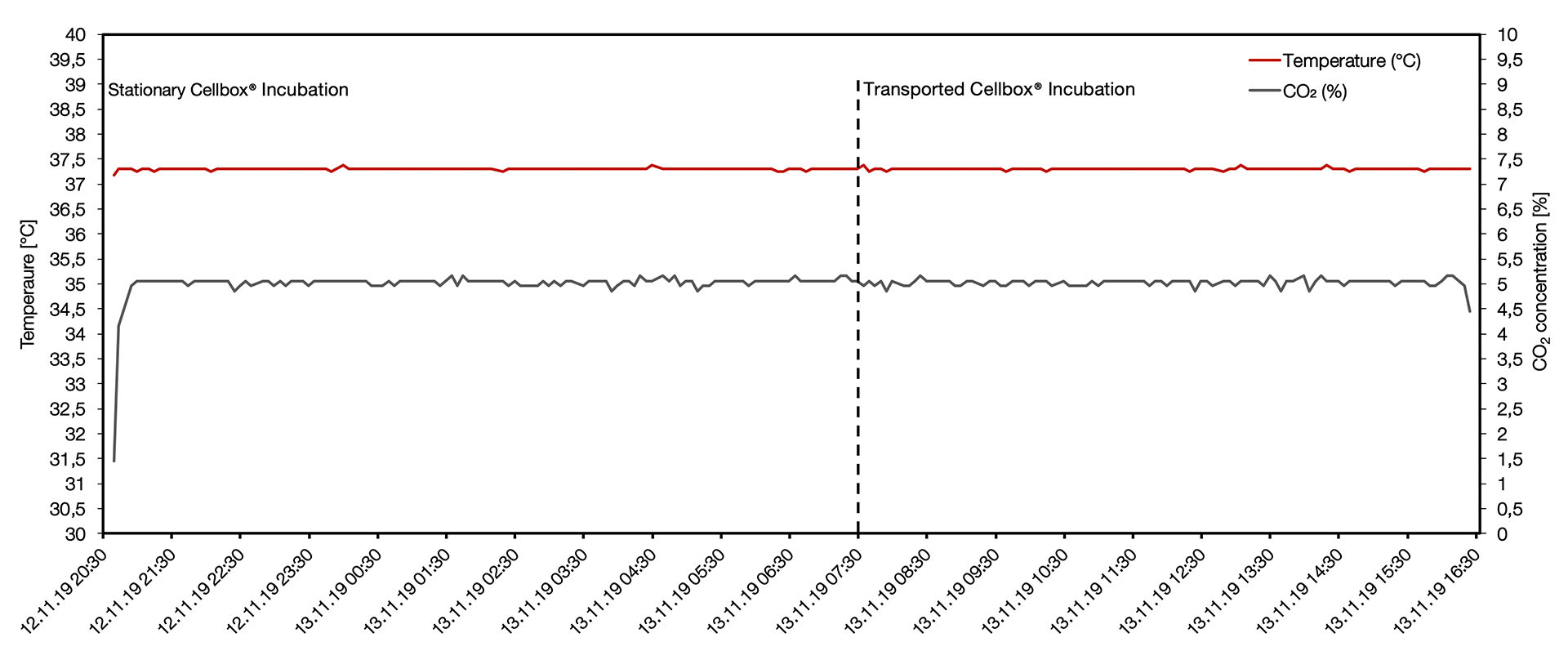
Comprehensive data logs are maintained by the Cellbox™ and can be exported following transport. These consist of measurements that are collected from the internal sensors at 5 minute intervals. Midbrain organoids were packaged in the Cellbox™ for overnight incubation before collection and transport for a period of 8 hours.
After the initial heating and CO₂ preconditioning phase the temperature was measured for 20 hours with an average temperature of 37.31 + 0.02 °C and an average CO₂ . concentration of 5.04 +0.06 %. No major deviations were observed during the transport.
Results: Cell Viability
Midbrain organoids were cultivated in cell culture media with and without GrowDex ®/GrowDex®-T to determine the influence of the artificial ECM on cell survival. The organoids were then either cultivated in a stationary incubator or transported in the Cellbox®. The cell survival rate was then measured by means of a CellTiterGlo3D assay.
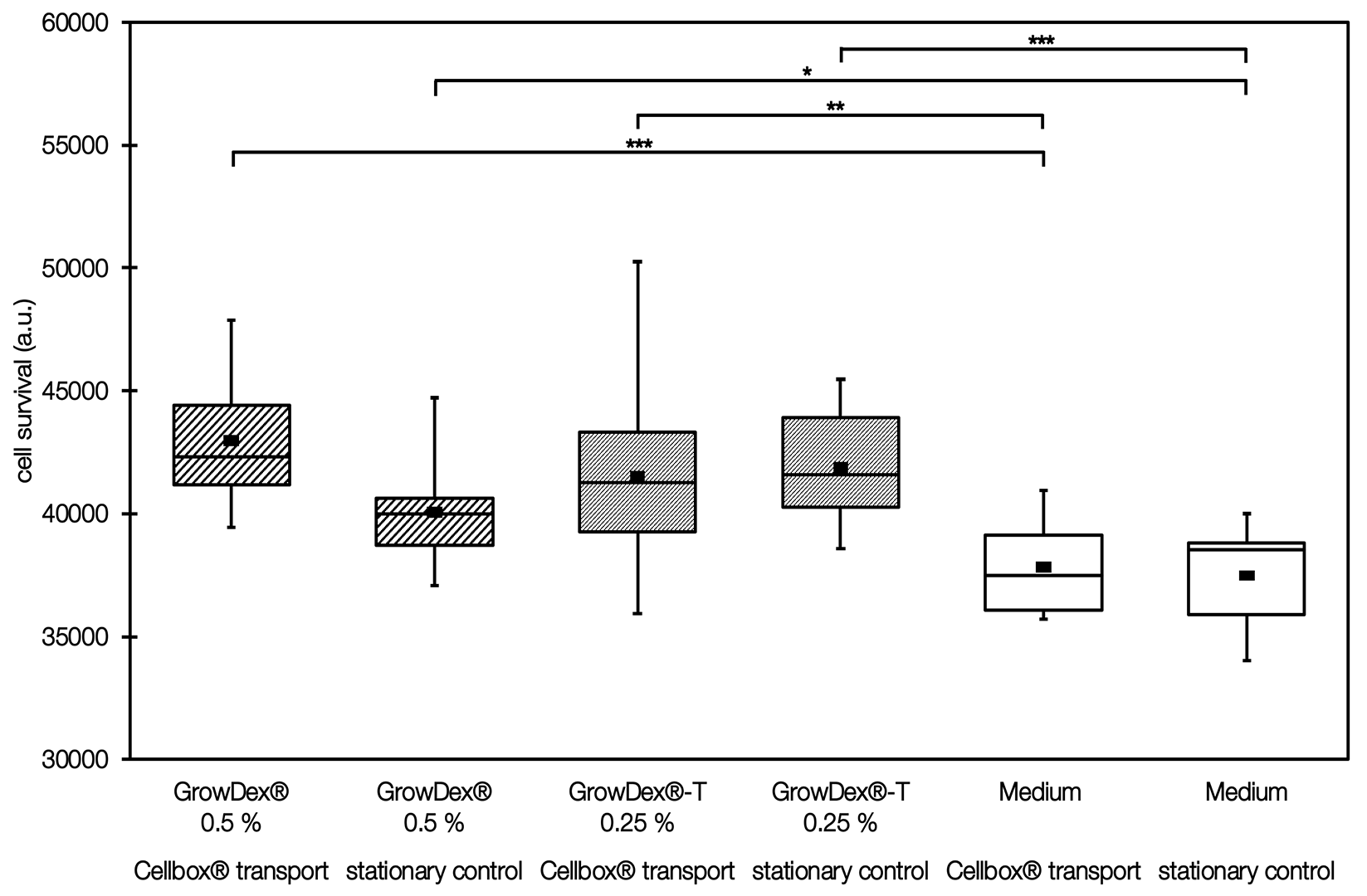
- Addition of GrowDex®-T 0.25 % and GrowDex® 0.5 % leads to increased cell survival in comparison to the medium only control.
- Transport accounts for only 1.6 % of the total variation observed between samples and is not considered to have a significant impact on the survival of cells. On the other hand, the addition of an artificial ECM has a beneficial impact on the organoids and accounts for 32.3 % of the variance in cell survival with a high significance (p<0.0001).
Conclusion
- ECM addition (GrowDex® @0.5 % and GrowDex®-T @0.25 % significantly increases the cell viabilty of midbrain organoids when compared to medium only control.
- Midbrain organoids are unaffected by transport in the Cellbox™ in comparison to stationary incubation in the laboratory. These organoids also benefit from an added ECM to have an optimal vivo-like environment during transport.
This project results from a cooperation between Cellbox Solutions GmbH, Hamburg, Germany
Max Planck Institute for molecular Biomedicine, Münster, Germany
UPM Biomedical, Helsinki, Finland

