Paolo Annibale Research Group |
University of St Andrews


Live Cell Imaging
How altered gravity affects cellular response to drugs
June 2024
How altered gravity affects cell signaling at the molecular level is a crucial question underpinning human health in space and it remains a largely unexplored domain. A large portion of extracellular signals in human cells are mediated by G protein-coupled receptors (GPCRs), a family of over 800 membrane proteins and a prominent drug target.
Their function is regulated by a complex network of extra and intra-cellular interactions, that is a prime candidate to be affected by the pleiotropic effects originating from altered gravity.
Alterations to GPCR-mediated signaling cascades can be observed at the level of second messenger production, i.e. cAMP for the canonical adrenergic stimulatory pathway. Changes in intracellular cAMP have broad consequences on cellular homeostasis, often cell-type specific, such as protein kinase A (PKA)-mediated increase in heart rate, immune regulation, chromatin condensation and regulation of intracellular transport.
Early reports have shown that cAMP homeostasis is affected in cellular organisms and single cells exposed to periods of altered gravity. We use biophotonics tools to measure response to drugs at the single cell level.
Our measurements have the potential for investigating a relatively unexplored field in space research, namely how pharmacology changes as a function of gravity with broad and important consequences for manned flight and space exploration at large.

The Project

Credits DLR (CC BY-NC-ND 3.0)
The project investigates cAMP levels in cells exposed to 1G or 0G for varying intervals ranging from 2 h to 24 h. We measured the cAMP response to adrenergic stimulation of endogenous β-Adrenergic Receptors (β-AR) in cardiomyocyte-like cells.
β-ARs, which are key GPCRs, increase cAMP levels via the Gs pathway upon agonist stimulation, e.g. increasing heart rate during the fight or flight response.
Altered gravity exposure was conducted at the German Aerospace Center (DLR) in Cologne using Micro-g lab clinostats or incubator centrifuges.
cAMP levels were measured with a Fluorescence Resonance Energy Transfer (FRET) biosensors based on the effector protein EPAC1.
Our over-arching goal is to understand how altered gravity affects molecular switches in the GPCR pathway to improve modulation and intervention in GPCR-mediated pharmacology in space.
The Outcome
Upon arrival, the cells were inspected using brightfield microscopy and lifted off the plates to create a single-cell suspension. These cells were plated in poly-L-lysine coated Ibidi micro-channel slides, suitable for fluid exchange during drug stimulation.
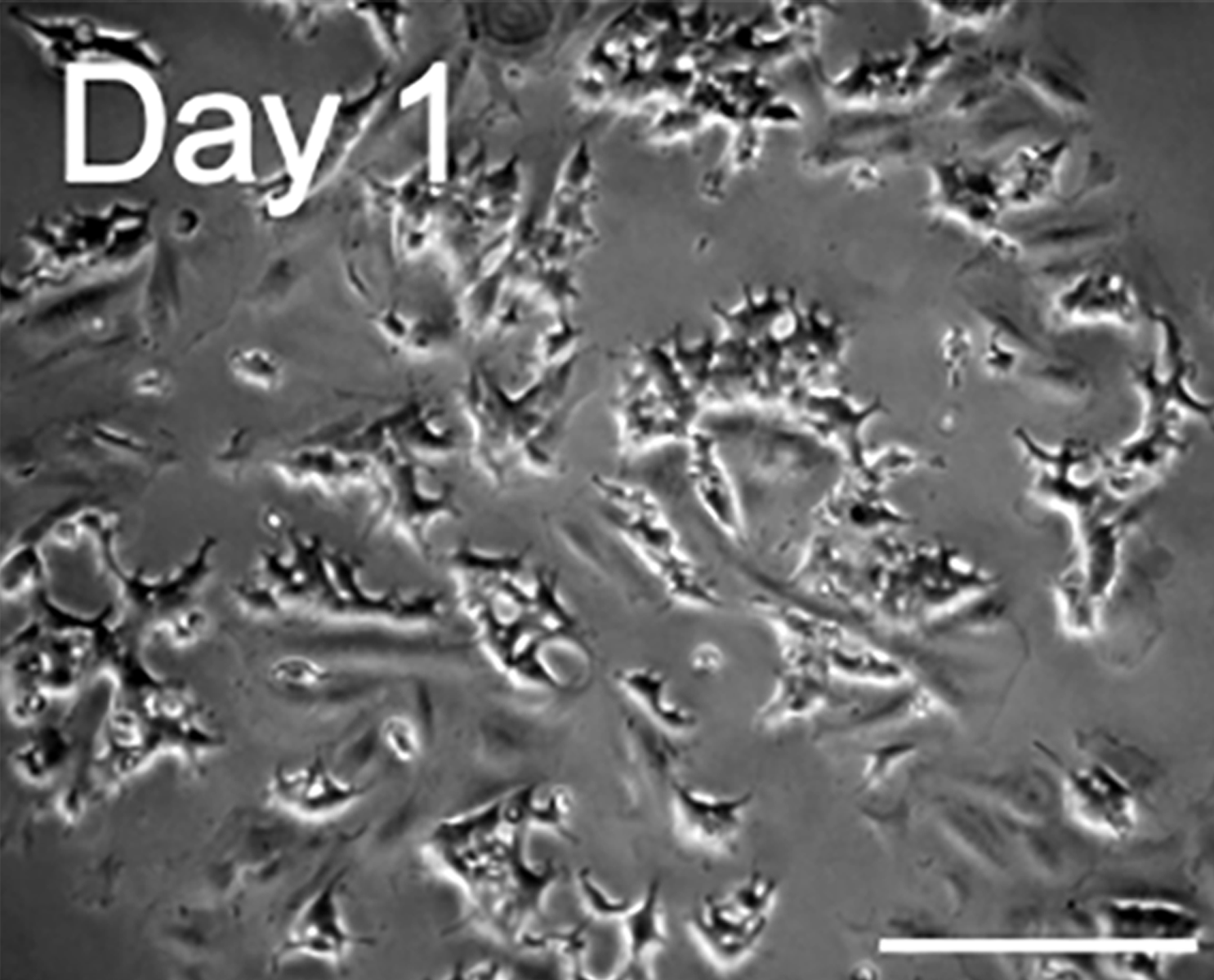
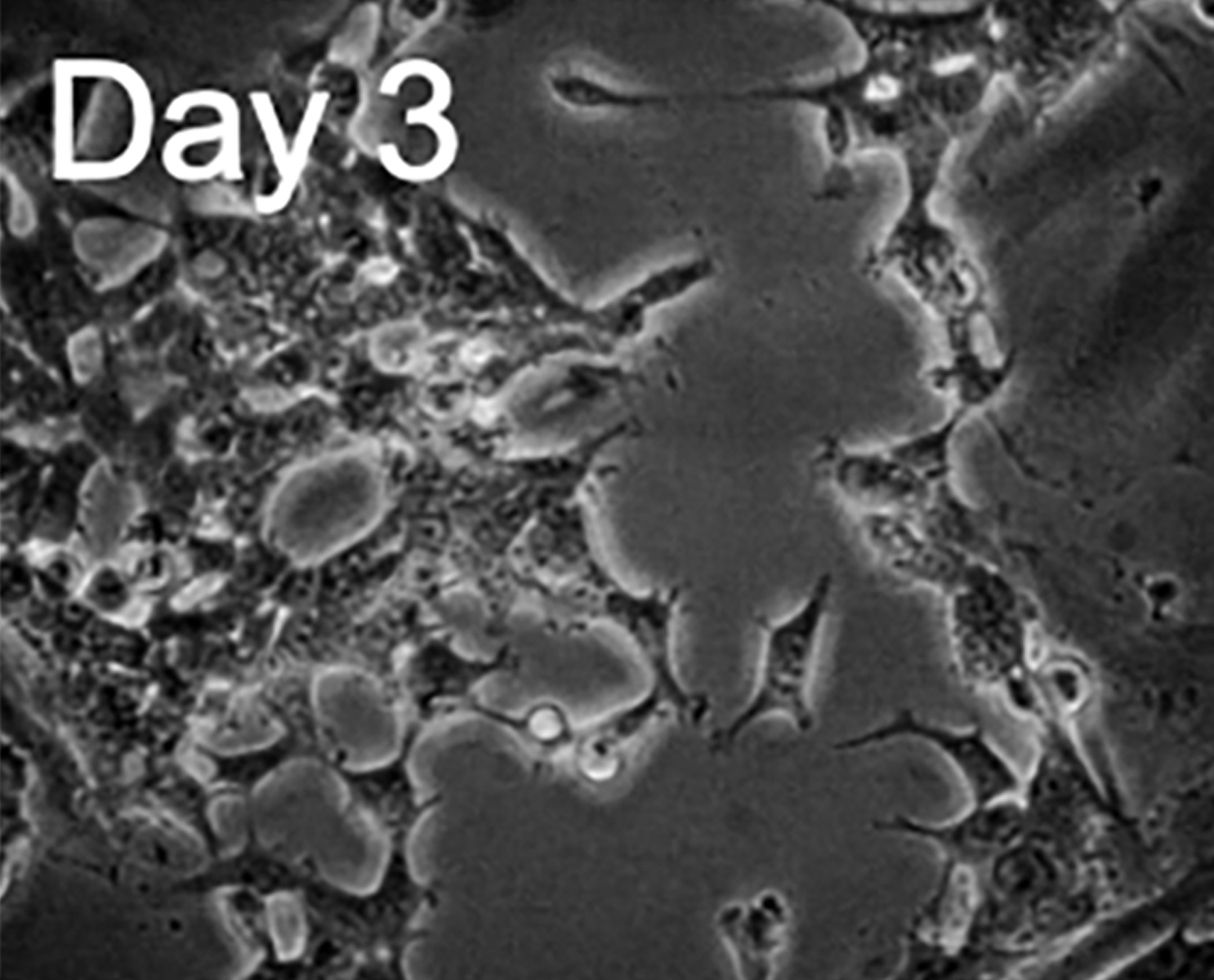
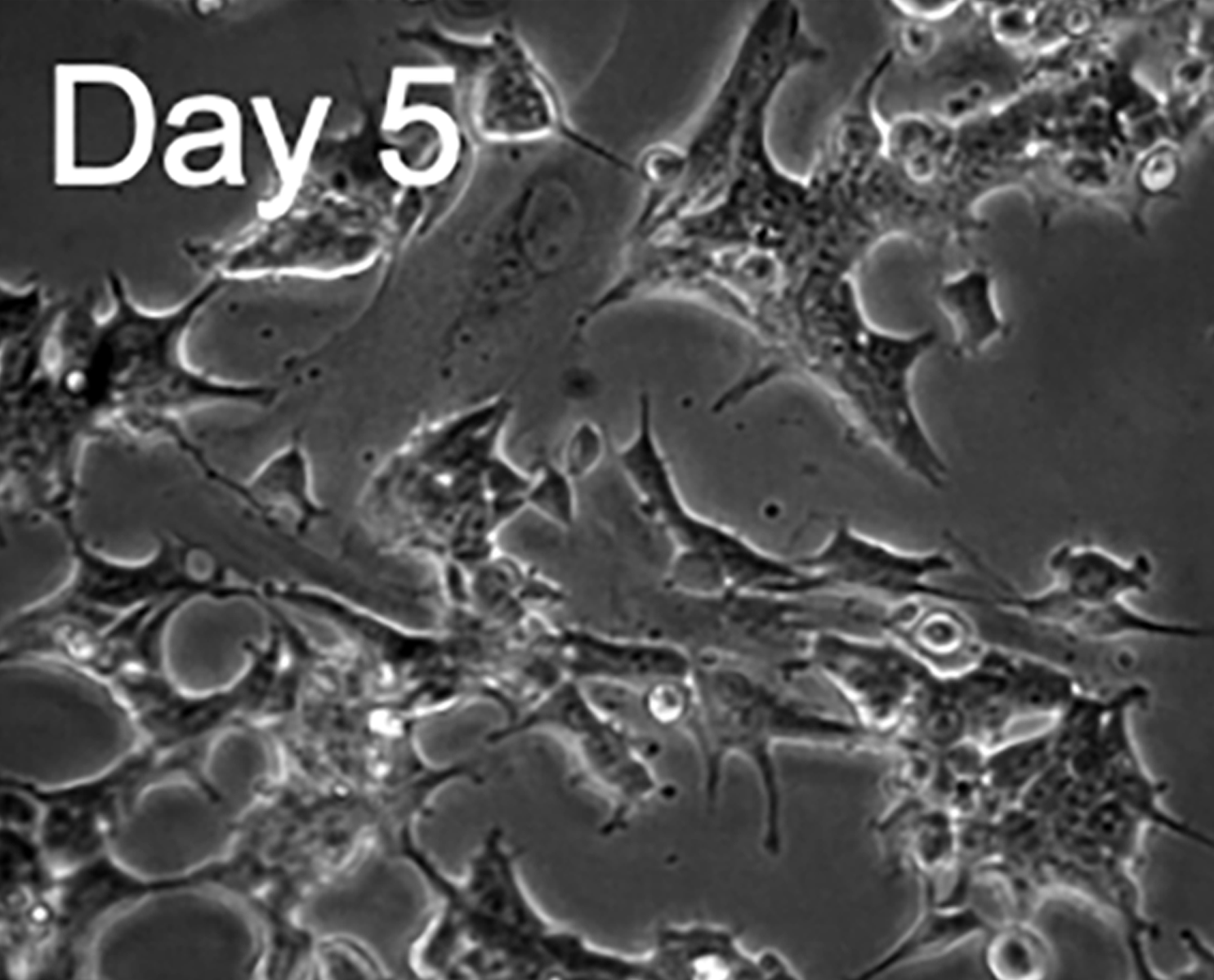
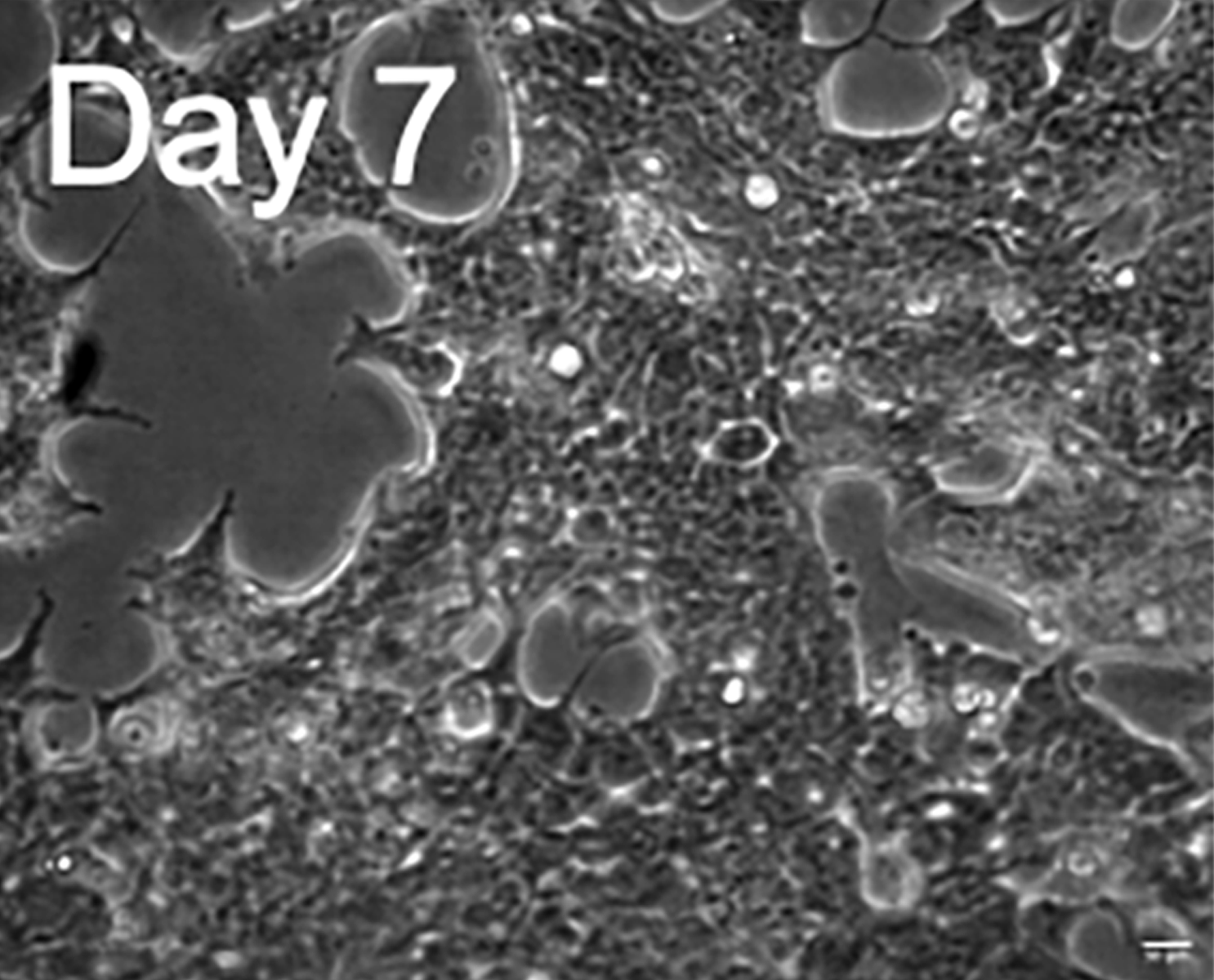
H9c2 rat myoblasts differentiated to a cardiomyocyte like state by incubation with retinoic acid for a week, and subsequently transfected with a FRET biosensor for cAMP detection.
Donor and FRET signals
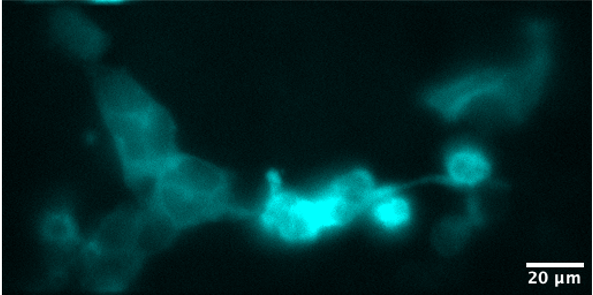
DONOR
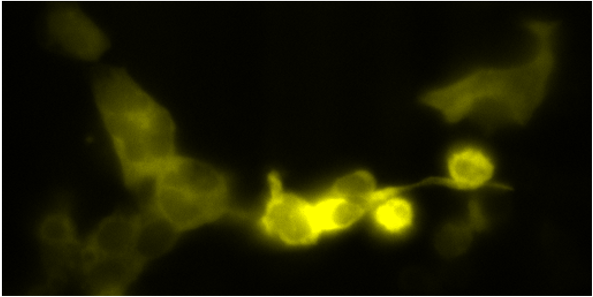
FRET
Donor and FRET signals were measured with an LED light source and using a motorised filter in the microscope turret. We generated kinetic traces showing the cells‘ response to adrenergic stimulation, which will be compared to the response under normal (1G) gravity. Subsequent analysis is ongoing.
Overall, the Cellbox™ has been an exceedingly useful asset, with its applications rapidly extending beyond its intended purpose.

The differentiated cells were transported using the Cellbox™ from our laboratory in St Andrews, Scotland, to the German Aerospace Center ground-based facilities in Cologne, Germany.
The cells were imaged using a live cell imaging microscope and a drug (isoproterenol) stimulating the endogenous β-ARs was flushed using a perfusion system while the microscope was spun on a centrifuge to achieve 2G conditions.
Our imaging experiments were successful, showing confluent and viable cells responding to adrenergic stimulation. (...)
This research was supported by the European Space Agency, the UK Space Agency, and Science and Technology Facilities Council
For more Information: Paolo Annibale Research Group



