a:head

ORGANOID
Functional Assessment of Brain Organoids After Transport
August 2025
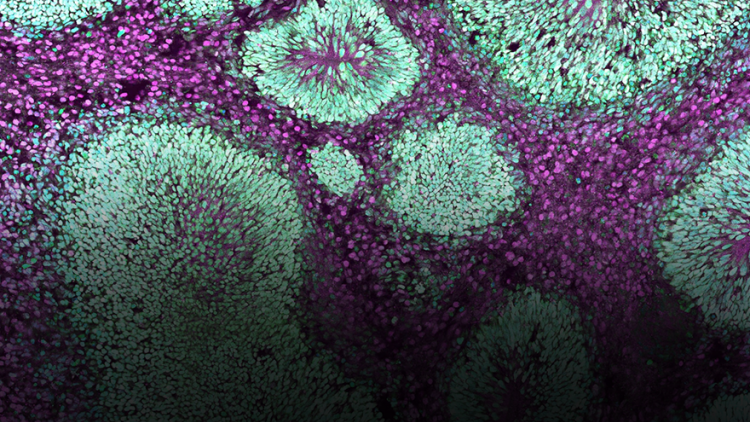
a:head bio AG develops human brain organoids to model CNS disease and advance drug discovery; in this use case, living organoids are shipped under warm-chain conditions and functionally validated on arrival.
The Project
This project aimed to evaluate the feasibility of transporting live 3D brain organoids using the portable CO₂ incubation system Cellbox™ Shipper under real-world conditions. The primary objective was to determine whether brain organoids could maintain their functional neuronal activity after transportation, thereby allowing for downstream pharmacological testing. The experiment was conducted in collaboration with a:head bio AG and combined controlled warm chain transport logistics with high-throughput functional imaging.

Transport Details
The brain organoids were transported by car from Vienna, Austria, to Würzburg, Germany, using the Cellbox™ Shipper system. The planned travel time was approximately 6.5 hours, but due to necessary recharging stops for the electric vehicle, the total journey lasted around 8.5 hours. Throughout the entire transport, the Cellbox™ maintained a stable internal environment with constant temperature and CO₂ levels, ensuring optimal conditions for the sensitive organoids. Upon arrival, the organoids were immediately processed for functional imaging without further delay.
Imaging Platform and Methodology
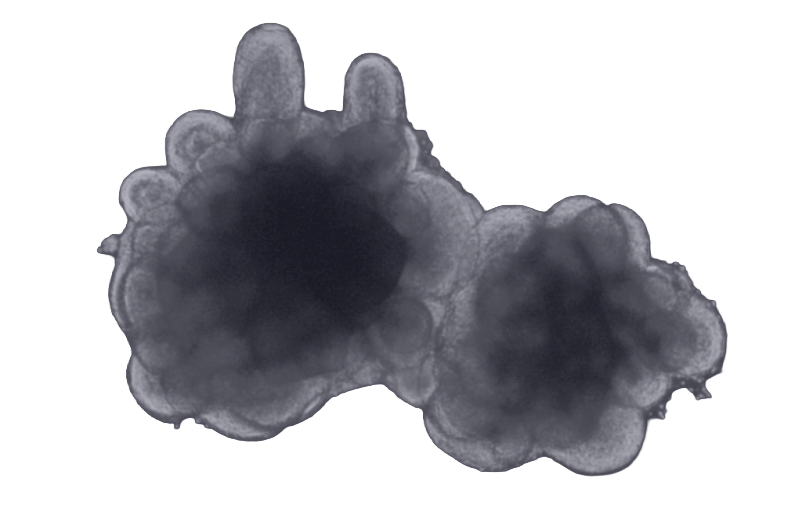
To assess neuronal activity, the FDSS / μCell system (Hamamatsu) was used. This high-throughput imaging platform captures calcium transients in real-time through fluorescence-based recordings. Organoids expressing the genetically encoded calcium indicator GCaMP were individually transferred to wells of a 24-well imaging plate. The system supports both acute and time-course recordings and is equipped with built-in temperature and CO₂ control.
A custom-developed Python analysis pipeline was used to identify organoids in each well, extract fluorescence time-series data, and quantify key calcium activity metrics that reflect the intrinsic neuronal functionality of the organoids.

Functional Readout Example
Post-transport recordings demonstrated that the organoids remained viable and exhibited active neuronal dynamics. Time-lapse videos of the entire plate confirmed structural integrity across all wells, while the calcium activity of a representative organoid revealed rhythmic and pronounced calcium transients. These observations validated the physiological state of the organoids after transport and confirmed the suitability of the model for further functional pharmacological analysis.
Compound Testing Strategy
To assess pharmacological responsiveness, a sequential application of increasing concentrations of neuroactive compounds was performed on individual organoids. Specifically, concentrations of 1 µM, 10 µM, and 100 µM were applied in sequence, with media changes in between. Neuronal activity was recorded after an incubation period with the compound, and changes were quantified using two metrics: number and mean amplitude of calcium transients. This approach enabled clear and reproducible assessment of the compound effects across multiple dose levels within the same organoid.

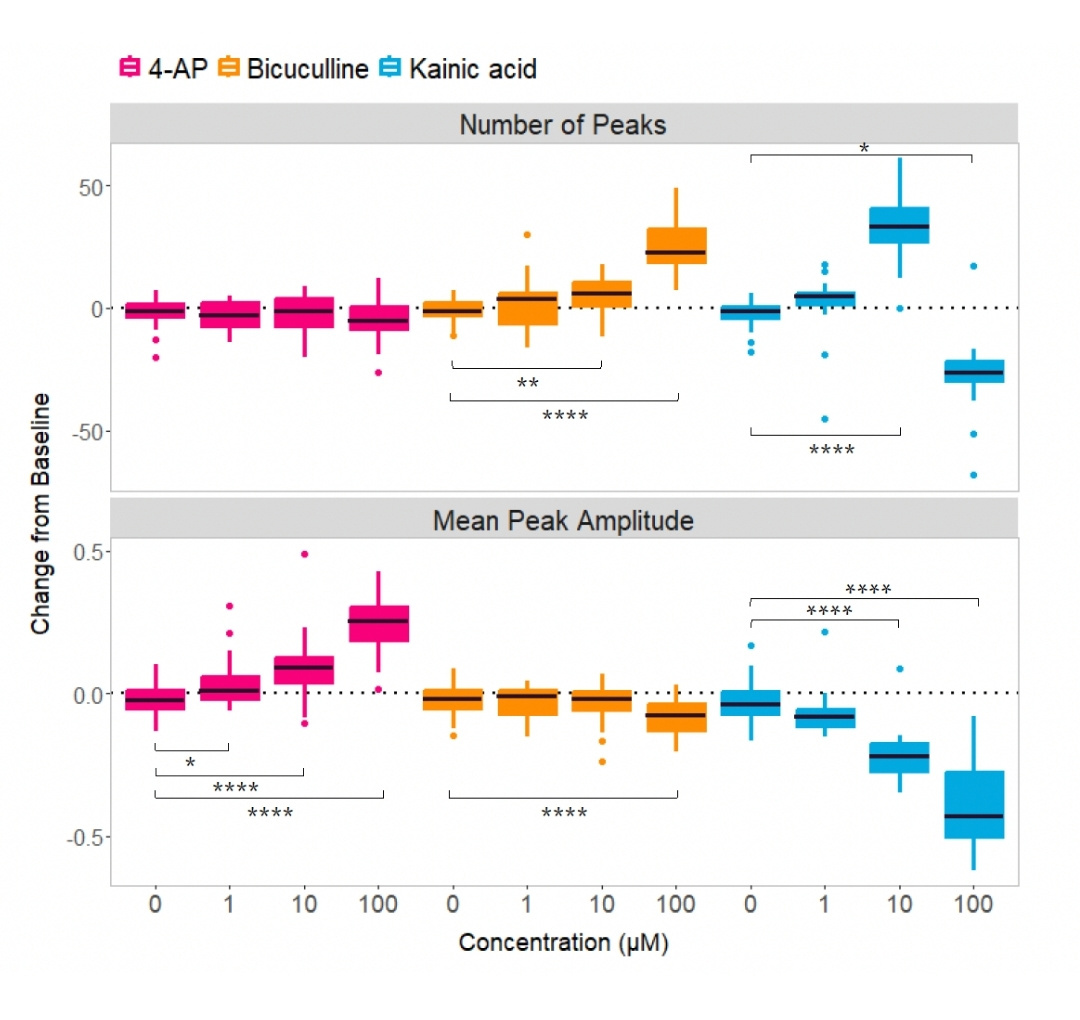
Results and Interpretation
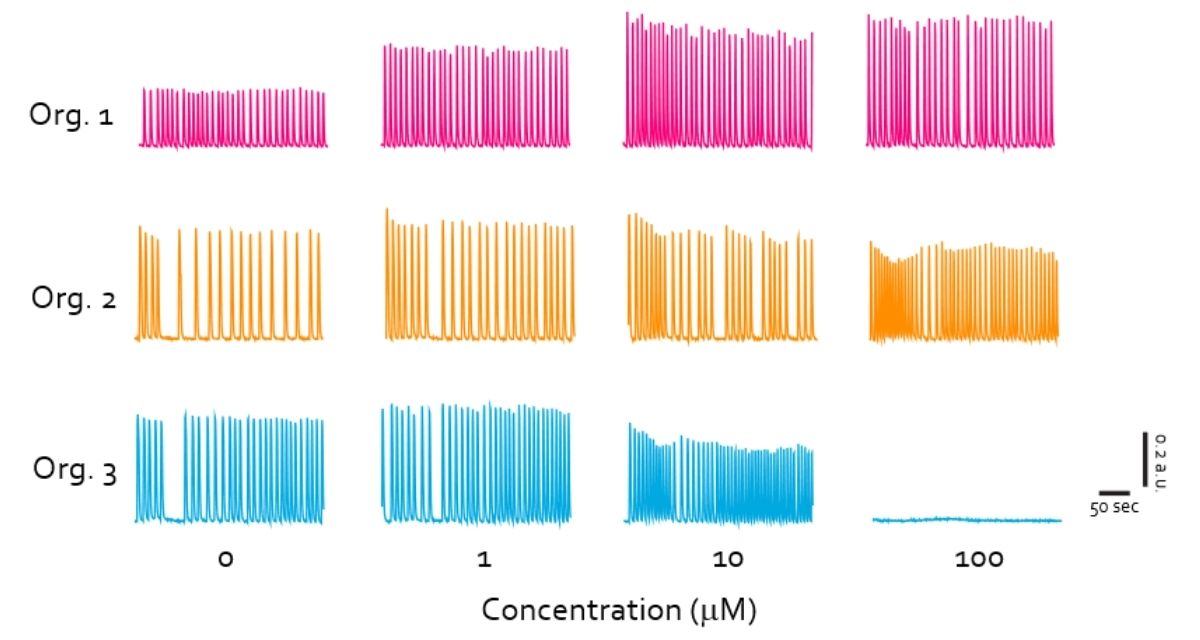
All three compounds tested - 4-Aminopyridine (4-AP), Bicuculline, and Kainic acid - produced dose-dependent effects on neuronal activity. 4-AP and Bicuculline altered network activity by modulating signal rate and amplitude in distinct, non-overlapping ways, reflecting their different mechanisms of action, whereas high concentrations of Kainic acid completely suppressed activity, consistent with its neuroexcitotoxic effects.
These findings were based on recordings from 39 organoids for 4-AP and Bicuculline, and 24 organoids for Kainic acid, using one cell line across two batches. Statistical significance was confirmed using the Friedman test for repeated measures followed by Dunn’s multiple comparisons test (p < 0.05 to ***p < 0.0001).
The Outcome
The results clearly demonstrate the feasibility of using the Cellbox™ system to transport live 3D brain organoids without compromising their functional integrity.
The organoids remained structurally intact and responsive to pharmacological stimulation, supporting the use of Cellbox™ as a reliable logistics solution for neurobiological research, compound screening, and preclinical evaluation involving complex brain tissue models.
Diverse set of convulsants impact on neuronal activity in a dose-dependent manner on brain organoids
Figure: Diverse set of convulsants impact on neuronal activity in a dose-dependent manner on brain organoids after transported in Cellbox™ for 6.5 hours. n = 39 organoids (4-AP and Bicuculline), n = 24 organoids (Kainic acid), 1 cell line, 2 batches.
| Compound | Class | Mechanism |
|---|---|---|
| 4-AP | Convulsant | Voltage-gated K+ channel blocker |
| Bicuculline | Convulsant | GABAa receptor antagonist; Competitive antagonist at GABA binding site |
| Kainic acid | Convulsant | Agonist of Kainate receptors and a partial agonist at AMPA receptors |
Dunn‘s multiple comparisons test
*, **, **** p < 0.05
n = 24 organoids (Kainic acid)
1 cell line
2 batches
Contact Persons:
a:head:
Bruno Fontinha
Cellbox Solutions:
Leye Coker

