VetEmbryo

IVF
In vitro maturation in a portable CO₂ incubator supports optimal embryo development
Aug 2023
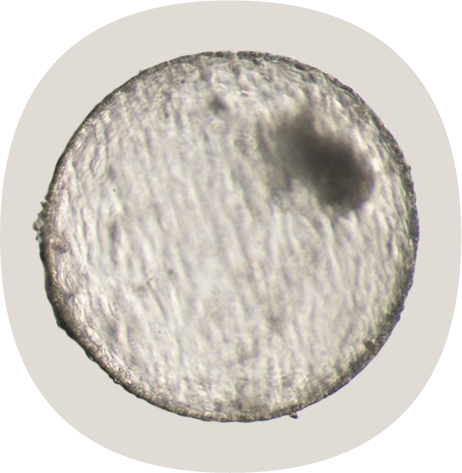
completely hatched blastocyst
The purpose of the initial part of this study was to evaluate standard quality control parameters, such as temperature, stability, gas concentration and VOC levels inside the chamber to evaluate if the Cellbox™ is accurate enough and non-toxic for the oocytes.
The purpose of the second part of this study was to investigate if it is possible to use the Cellbox™ Ground Shipper for CO₂ equilibrated in vitro maturation (IVM) of bovine oocytes as the initial step of in vitro fertilization (IVF) without detrimental effect on IVF embryo development.
As an additional group, we have added a group of oocytes matured in hepes buffered medium in the Cellbox™ chamber, as transport of oocytes in hepes buffered medium is the alternative method to transport during CO₂ equilibration.
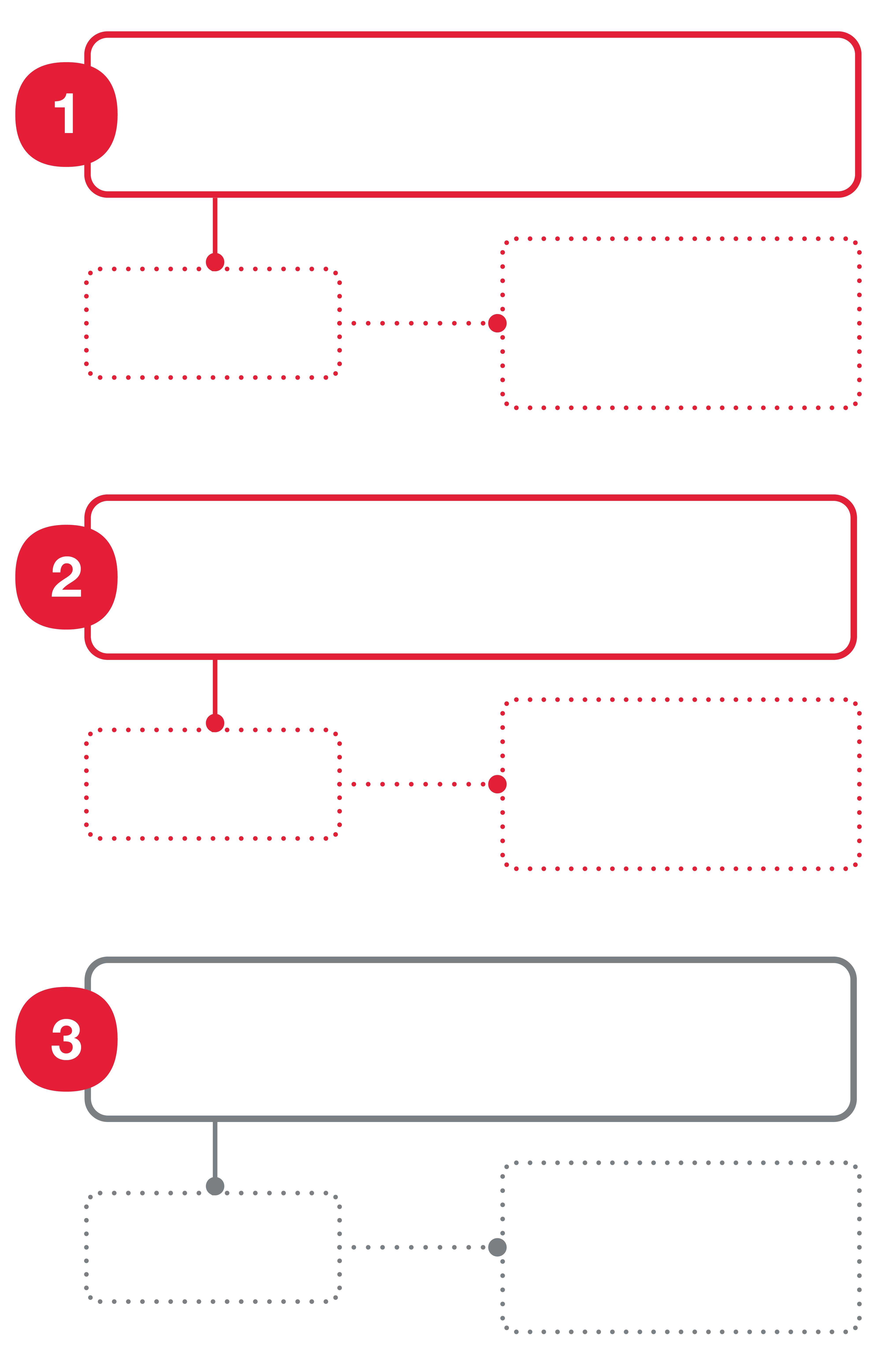
Figure 1
Diagram to illustrate the 3 study groups. The main comparison is between maturation in CO2 equilibrated medium in the Cellbox™ and conventional CO2 in vitro maturation (IVM).
The Cellbox™ hepes-IVM group is added as a secondary comparison between CO2 equilibrated medium and hepes buffered medium in the Cellbox™, as hepes-buffered IVM is the alternative to CO2 equilibrated IVM during transport.
Materials and Methods
Picture from VetEmbryo
2.1 Collection of cumulus-oocyte complexes (COC) and in vitro maturation (IVM)
All media were from IVF Bioscience, United Kingdom, unless otherwise stated (https://ivfbioscience.com/bovine-media/ ). Bovine ovaries were collected at a local abattoir (Danish Crown, Holsted).
VetEmbryo holds an art. 17 permission to collect oocytes and use them for research purposes in the laboratory. The ovaries were transported to the laboratory in physiological saline (0.9%) at ~ 30°C within 3 h after slaughtering.
Cumulus-oocyte complexes (COCs) were aspirated from 2 to 150 mm diameter follicles using 18G needles attached to a vacuum pump. The oocytes were washed three times in Wash and one time in IVM medium, mixed, and randomly distributed into groups of oocytes going into each well.
In vitro maturation was performed with BO-IVM medium, and oocytes were incubated in groups of 30-45 oocytes in one well/vial with 500-1000 μl BO-IVM medium at 38.0-38.5°C and 6% CO₂ in atmospheric air for 21-24 hours.
Picture by VetEmbryo

2.2 Spermatozoa preparation
Frozen semen from two bulls (Noble and Calvin) of known in vivo fertility was used for this study. Ten straws were thawed and mixed with an equal volume of BO-SEMENPREP (1:1 dilution). 850 μl of this suspension were loaded on the Bovine VetMotl multi (850 μl) sperm separation device (VMB0850 https://vetmotl.com/ ) inlet with a MEA tested 1 ml syringe. Hereafter, approximately 750 μl of warm media (BO-SEMENPREP) were added onto the membrane surface and the outlet port was primed with media.

The WTA tubes used for CO2 maturation in the Cellbox™ chamber
The chip was then incubated in a humid incubator at 38 °C for 30 minutes. After 30 minutes, 500 μl sperm-containing media were collected from the outlet and added to one tube and centrifuged at 300 g for 5 minutes and the supernatant was removed down to the 200 μl line.
The pellet was resuspended, and the concentration was determined with a Makler counting chamber.
2.3 In vitro fertilization (IVF) and culture (IVC)
The COCs were evaluated for cumulus expansion and viscoelasticity and then washed in BO-IVF medium. The group of COCs were placed in a well with 400 μl BO-IVF medium in a volume of 50 μl. The sperm suspension was then added at approximately 50 μl to obtain a final volume of 500 μl. In total approximately 0,5106 spz were added to the well, reaching a final concentration of 1106 spz/ml. COCs and spermatozoa were co-incubated at 38.5 °C and 6 % CO₂ in atmospheric air for 16-20 h.

Picture by VetEmbryo

Picture by VetEmbryo
Fertilized oocytes were denuded by vortexing in Wash medium at full speed for 2 minutes. Then they were washed three times in Wash medium and one time in BO-IVC medium. Fertilized oocytes were then cultured in BO-IVC medium at 38.5°C in 6% CO₂, 5% O₂ in 89% N₂.
On the 2nd day of culture (48 hours after insemination) we registered the number of cleaved embryos, on the 7th and 8th day the number of blastocysts, and on the 9th day the number of hatched blastocysts.
2.4 Statistical analysis

Initial calculations were performed in Excel and all statistical analysis were performed in GraphPad Prism.
For comparisons of proportions (rates), we used Fisher’s exact test with a threshold of 0.05 for significance.
In Vitro Fertilization
Figure 3 Diagram illustrating the study setup for the in vitro embryo production study. Created with biorender.com.
Results and discussion
3.1. Initial quality assurance of the Cellbox™
The aim was to evaluate the suitability and safety of the Cellbox™ Shipper Ground for transported oocyte IVM.
Temperature logging in the Cellbox™ chamber. Considering the measurement accuracy of the temperature logger, the chamber temperature is very stable in an acceptable range between 37.8°C and 37.9°C.
Temperature stability


Measurements of the temperature inside the chamber with surface temperature probes:
- Instrument: NiloChecker with dual digital temperature probe from Nilotech (0.03°C accuracy)
- Temp: probe 1 = 38.14 °C and probe 2 = 37.86 °C
- Cellbox™ was set at 38.0 °C
- Conclusion: passed.
Temperature logging over time to evaluate temperature stability over time
- Logger: Omega, OM-91, data logger, temperature
- Cellbox was set at 38.0 °C
- Conclusion: From the temperature graph (Figure 5) the chamber-temperature is a bit over-shooting in the beginning and then it stabilizes at 38.16 °C for a very long time and as the accuracy of the temperature logger is ± 0.3 °C the stabilized temperature is within the desired range.
Figure 6:
The ION CUB gas analyzer measuring VOCs and the temperature logger device
inside the chamber.

→
Measurement of the total VOC levels inside the chamber:
- Instrument: ION CUB 10.6eV gas analyzer (Figure 6).
- Result: 0,000 to 0,004 ppm (during 6% CO₂ and 38.0 °C).
- Conclusion: passed.
Measurement of the CO₂ gas concentration inside the chamber:
- Instrument: NiloChecker from Nilotech, DG 112 Digital Carbon Dioxide(CO₂) and Oxygen (O₂) probe with an accuracy of 0,2% O2 and 0,1% CO2 (Figure 4).
- CO₂: 6.1 %
- Conclusion: passed.
Measurement of the pH in medium, equilibrated in the Cellbox CO₂ chamber:
- Instrument: epoc Vet, blood-gas-analyzer
- Desired range: pH 7.2-7.4
- Measurements on BO-IVM medium equilibrated overnight in WTA tubes in the Cellbox chamber at 6% CO2:
> pH: 7.366 > pCO2: 40.5 mmHg > pO2: 136.9 mmHg > TCO2: 23.5 mmol/L - Conclusion: all values are within the desired range.
Bovine in vitro embryo production study
During maturation all oocytes were randomly assigned to one of the 3 groups (Table 2, column 2). After maturation for 21-24 hours, the oocytes were moved to IVF medium and mixed with purified spermatozoa.
After 16-20 hours of co-incubation with spermatozoa, the oocytes/ presumptive zygotes were washed, denuded, and moved to culture medium.
This study was performed with one day of oocyte collection: Thursday 20th June 2024.
We collected in total 753 oocytes.
Picture by VetEmbryo
At 48 hours after the onset of insemination (after approximately 28 hours in culture medium) the oocytes/zygotes were evaluated for cleavage. We found no significant difference in cleavage rate (total cleaved zygotes/total oocytes) between the three groups (Table 2, Column 3 and Figure 7).
The cleavage rate was satisfactory in all groups indicating a good level of oocyte maturation and a good fertilization rate.
On day 7 of culture
(after insemination), the number of total blastocysts were counted (blastocysts, expanded blastocysts and hatched blastocysts) and the blastocyst rate was calculated (total number of blastocysts/total number of oocytes). We found no significant difference in blastocyst rate between the three groups (Table 2, column 3 and 4 and Figure 7).
On day 9 of culture
(after insemination), we evaluated the hatching rate (total number of hatched blastocysts/total number of blastocysts). We found no significant difference in hatching rate between the three treatment groups (Table 2, column 6 and Figure 7).

Figure 7
Embryo development in the study was evaluated through the four described parameters: cleavage rate on day 2, blastocyst rate on day 7 and 8 and hatching rate on day 9.
All treatment groups performed well and we found no significant difference between treatment groups for the four outcome parameters. Statistics: Fisher's exact test, p>0.05.

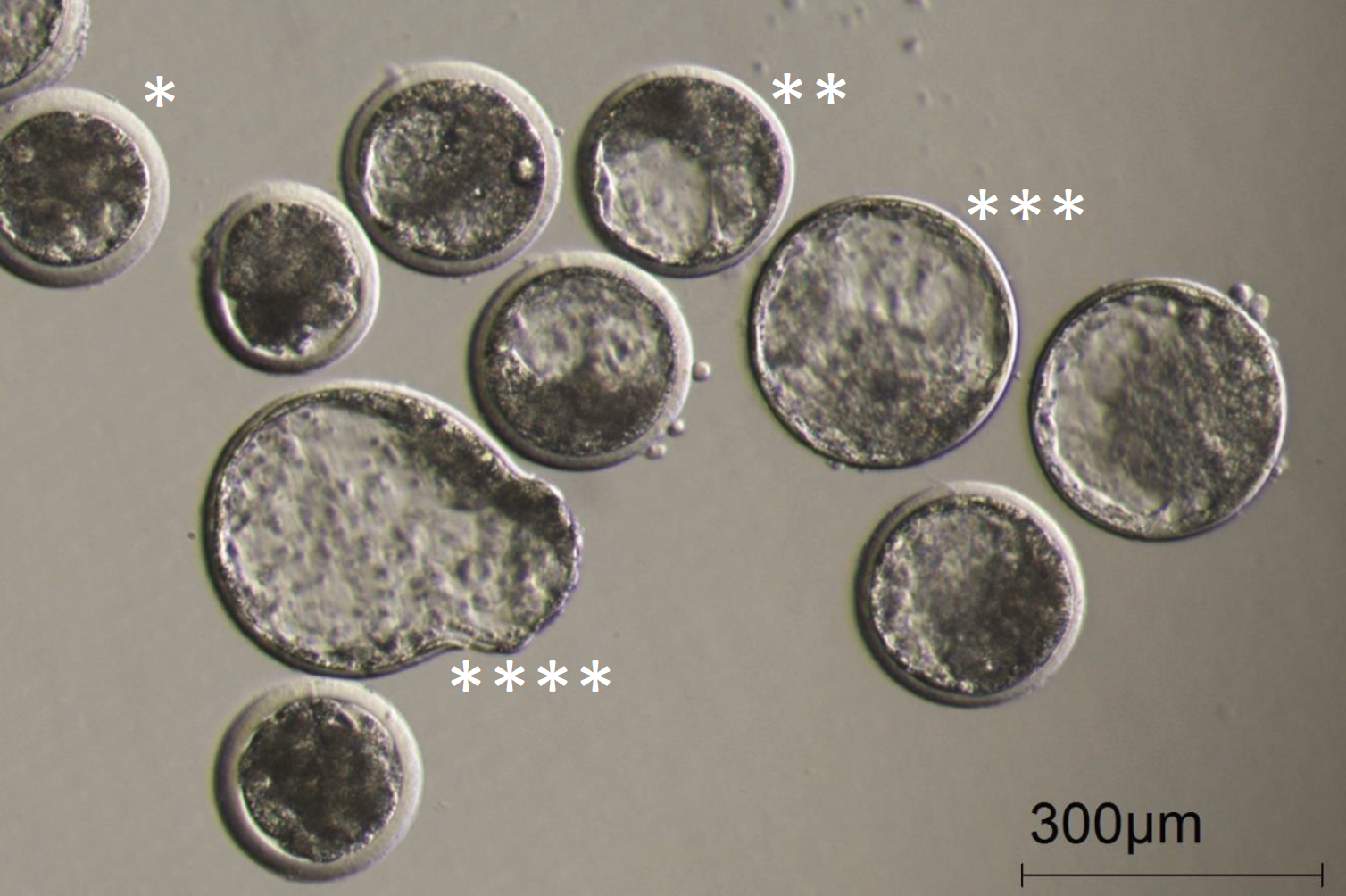
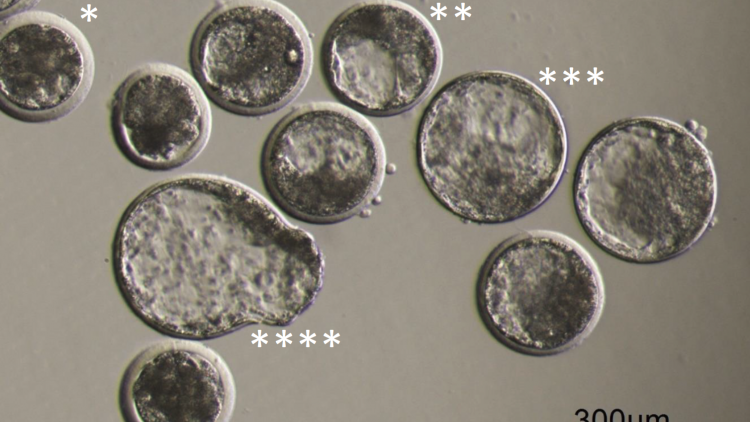
Figure 8
A picture with a collection of day 7 embryos made from oocytes matured in the Cellbox™ Shipper Ground. Examples of the different types of blastocysts are marked with asterisks:
* Early blastocyst
** Blastocyst
*** Expanded blastocyst and
**** Hatching blastocyst
Figure 9
A completely hatched blastocyst from an oocyte matured in the Cellbox™ Shipper Ground
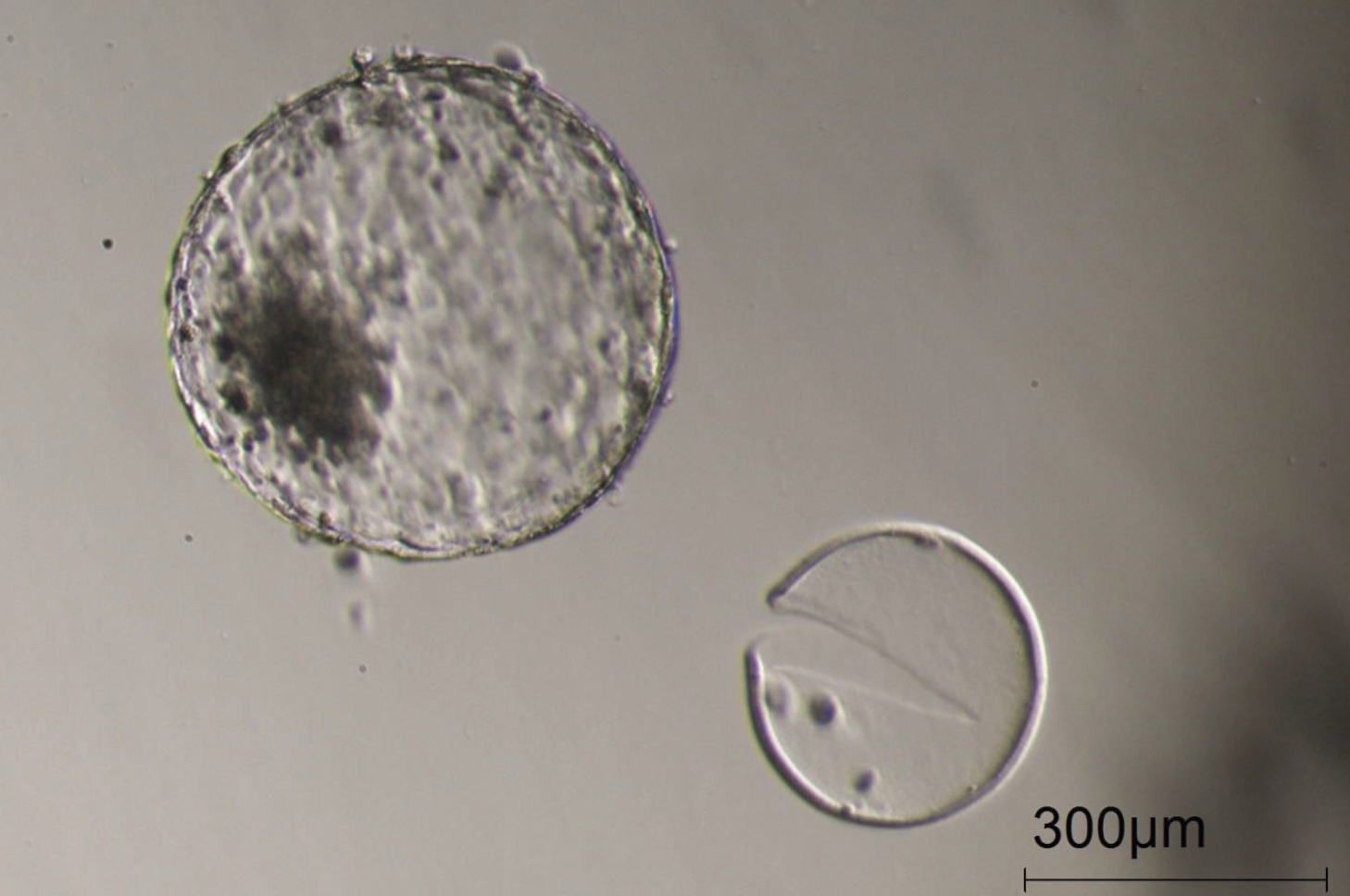
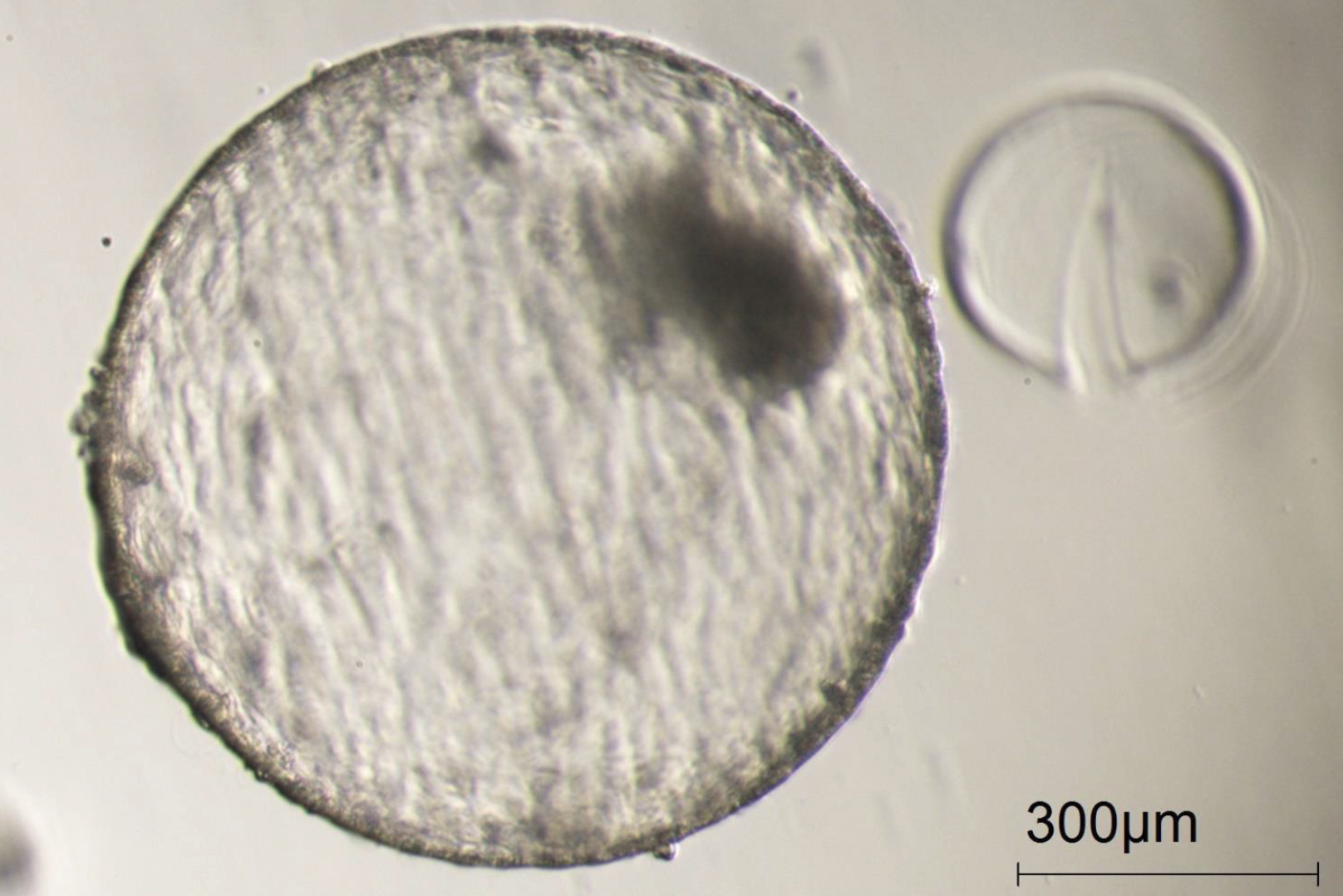
Figure 10
A completely hatched blastocyst, growing very big in vitro, just one day later than in Figure 9, illustrating the viability of the blastocysts created with oocytes matured in the Cellbox™.
Conclusions

Initial quality assurance evaluations of the Cellbox™ chamber temperature and gas concentration, VOC levels, and pH in the medium, confirmed that the Cellbox™ Shipper Ground supports accurate, safe and stable conditions for in vitro oocyte maturation in CO₂ equilibrated medium.
The in vitro embryo production study showed that oocytes matured in vials with CO₂ equilibrated medium in the Cellbox™ Shipper developed embryos at completely similar rates to our conventional system, confirming that the Cellbox™ supports oocyte maturation and embryo development at good rates and that the produced embryos are of good quality with similar in vitro viability to embryos developed from conventional maturation.
The hatching rate was numerically higher in the Cellbox CO₂ group (65%) compared to the Cellbox™ hepes group (52%) (Figure 7), however, the difference was not significant (Fisher’s exact test, p>0.05). The group of oocytes in the Hepes IVM group was rather small, as this comparison was secondary to the main comparison of Cellbox™ CO₂ maturation and conventional CO₂ maturation.
Further studies would be needed, to elucidate if there is a true difference and clear benefit from CO₂ equilibrated medium maturation compared to hepes-buffered maturation medium.
In cooperation with VetEmbryo and Cellbox Solutions.

