3Brain AG

ORGANOID
Advancing Neuroscience Research Through Safely Delivered Collaboration
July 2025

Innovative research relies on collaboration, bringing together expertise from diverse fields to tackle complex scientific challenges. Whether within academia, between academia and industry, or among industry themselves, such partnerships drive scientific progress.
One advancement that could benefit laboratories worldwide is the ability to transport delicate, complex cellular models between research facilities without the need for freezing down samples, a process which can alter their properties.
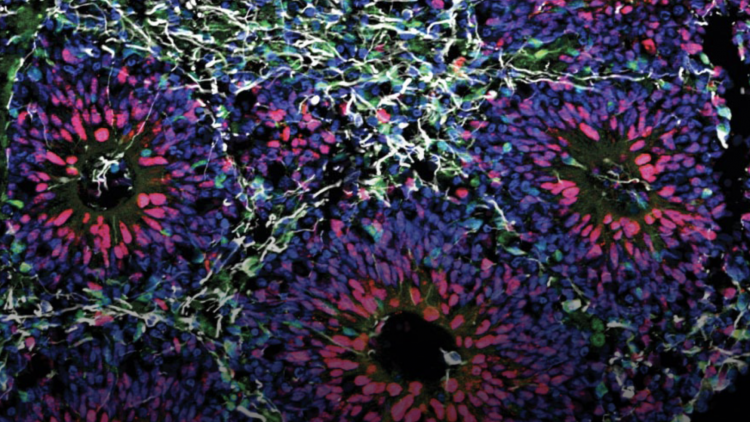
Among these sensitive biological models are brain organoids, which require strict conditions to maintain their viability. Ensuring they remain in their optimal conditions during transport is essential for conducting accurate and reliable research, and this is where the recent collaboration of 3Brain with Cellbox™ has proven invaluable.


CorePlate™ 1W 38/60
As a leading High-Density Microelectrode Array (HD-MEA) developer, 3Brain provides high-quality HD-MEA platforms powered by CorePlate™, enabling precise, high-resolution electrophysiological recordings. However, access to biological models can sometimes be limited by location. For instance, while 3Brain is headquartered in Switzerland, there are additional branches in Italy and the United States. The Italian lab plays a central role in supporting other 3Brain facilities by providing a wet lab facility for culturing and testing biological models, and providing demonstrations.
In this context, the collaboration with Cellbox™ enabled the safe shipment of organoids and spheroids from the 3Brain Italian lab, to the 3Brain HQ in Switzerland, preserving cell viability and enabling high-quality electrophysiological assessment of the organoids at the HQ after transport.
The Project
The organoids and spheroids were cultured at the 3Brain Genova facility (Italy), and upon reaching maturity they were shipped to 3Brain Pfäffikon SZ (Switzerland).
Once received, electrophysiological recordings were conducted using 3Brain’s HD-MEA platform, the BioCAM Duplex, in combination with CorePlate™ 1W 38/60.
This setup allowed the capture of vast amounts of precise, high-quality data, offering detailed insights into the functional activity of the organoids following transportation.

BioCAM Duplex
The Outcome
The successful delivery of organoids and spheroids without compromising their physiological integrity was a major achievement. Electrophysiological recordings confirmed that the organoids exhibited sustained spontaneous activity (Fig. 1) with robust functional connections spanning all over the organoids (Fig. 2) and showed no signs of impairment from the transportation process. Similarly, the spheroids maintained their viability, displaying spontaneous activity post-transportation (Fig. 3).
This not only highlights the reliability of Cellbox’s transport system but also the broader potential of such collaborations in expanding neuroscience research. By enabling the seamless exchange of complex biological models between laboratories, this approach paves the way for more extensive, multi-site research efforts, ultimately accelerating discoveries in neuroscience.
Electrophysiological traces:
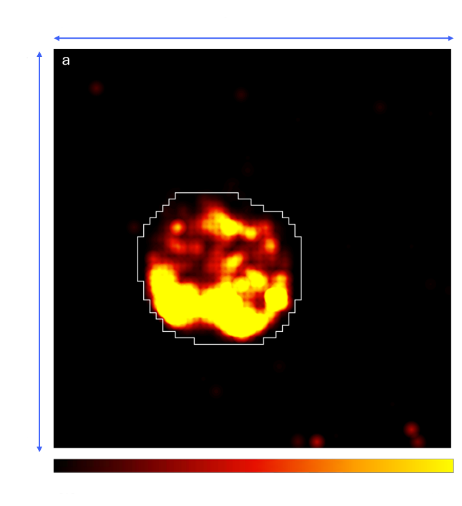
Figure 1 (A)
Figure 1 (A)
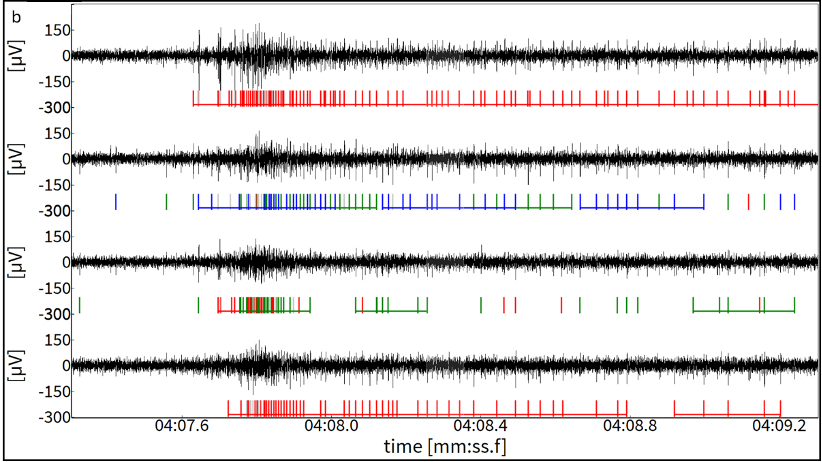
Figure 1 (B)
Figure 1. (A) Activity Map displaying spontaneous spiking activity within the organoid area (outlined in white), recorded after delivery. 429 electrodes showed a firing rate > 0.1 spikes/sec. (B) Raw electrophysiological traces from four recording channels. Detected spikes are indicated by vertical lines beneath each trace. These underwent spike sorting, therefore activity from different neuronal sources within each trace are represented by different colors. Bursts of spikes are indicated by horizontal lines over the spikes, highlighting periods of rapid firing
Metrics of organoid activity:
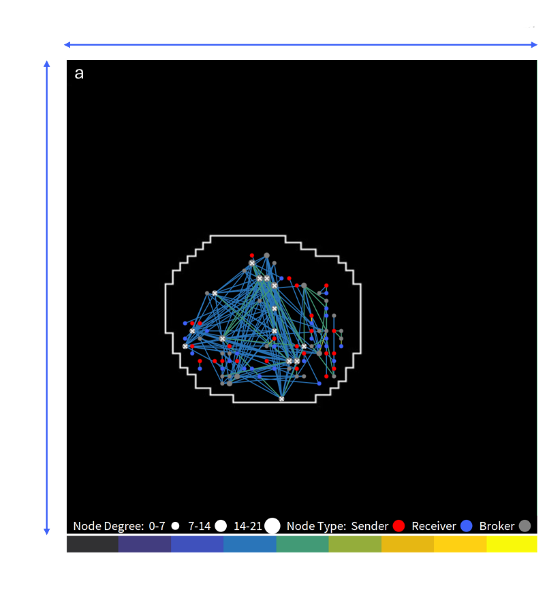
Figure 2 (A)
Network connectivity map displaying functional connections within the organoid (outlined in white) after delivery.
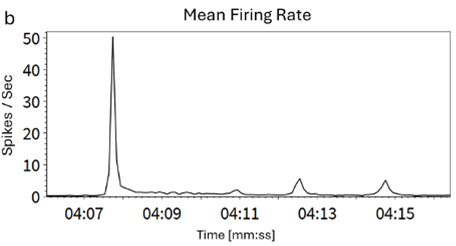
Figure 2 (B)
Firing rate trend over 10 seconds during network burst activity averaged on all active electrodes.
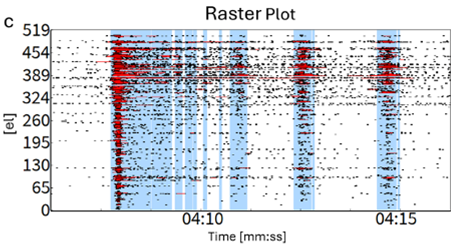
Figure 2 (C)
Raster Plot of the active electrodes displaying spikes (black dots), spike bursts (red horizontal lines) and network bursts (vertical blue lines).
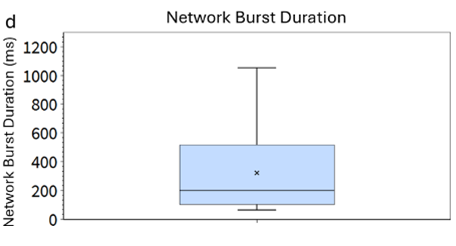
Figure 2 (D)
Network Burst Duration
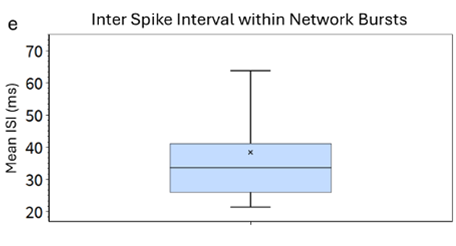
Figure 2 (E)
Inter Spike Interval within Network Bursts.
The Spheroids raster plot:
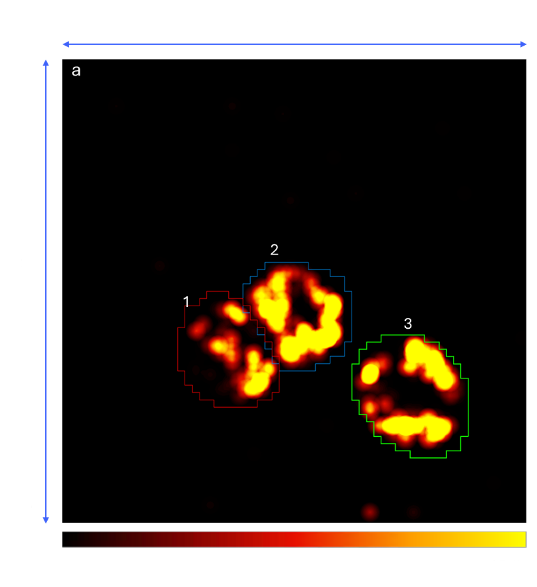
Figure 3 (A)
Figure 3 (A)
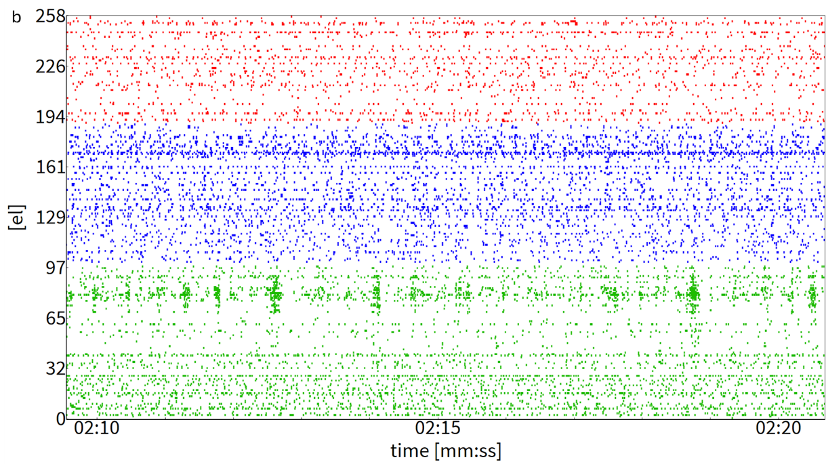
Figure 3 (B)
Figure 3. (A) Activity Map displaying spontaneous spiking activity from three spheroids (outlined in colored circles) recorded simultaneously on the same chip following delivery. (B) Raster plot showing a representative 10 second recording of the active electrodes. Spikes from each spheroid are color coded (red el=69, blue el=90, green el=99: all with firing rates > 0.1 spikes/sec)

