October 2025
The Silent Revolution: How Mesenchymal Stem Cells (MSCs) Are Redefining Regenerative Medicine
Written by: Robin Sieg & Prof. Dr. Kathrin Adlkofer
From the first cell cultures to clinical breakthroughs – a look at the current state of research, global trends, and the (so far) invisible role of logistics.
As so often in science, it begins with a quiet discovery: In the 1970s, researchers observed a cell population in bone marrow that differed from everything known at the time. It was neither a blood stem cell nor an immune cell, but a malleable, regenerative tool of the body. These cells could transform into bone, cartilage, or fat and were surprisingly easy to cultivate.
Today, half a century later, mesenchymal stem cells (MSCs) stand at the center of modern regenerative medicine. Worldwide, more than 1,670 clinical studies are underway testing MSCs, from treating inflammatory diseases to repairing heart, lung, and neural tissue.¹ Their versatility captivates both researchers and companies.
In contrast to embryonic stem cells, which can theoretically differentiate into any cell type, MSCs are “only” multipotent: they have a defined repertoire, such as forming bone, cartilage, or fat. That may sound like a limitation, but it is precisely this specialization that makes them so valuable in medicine. MSCs are biologically resilient, with minimal safety concerns and clear ethical acceptance.
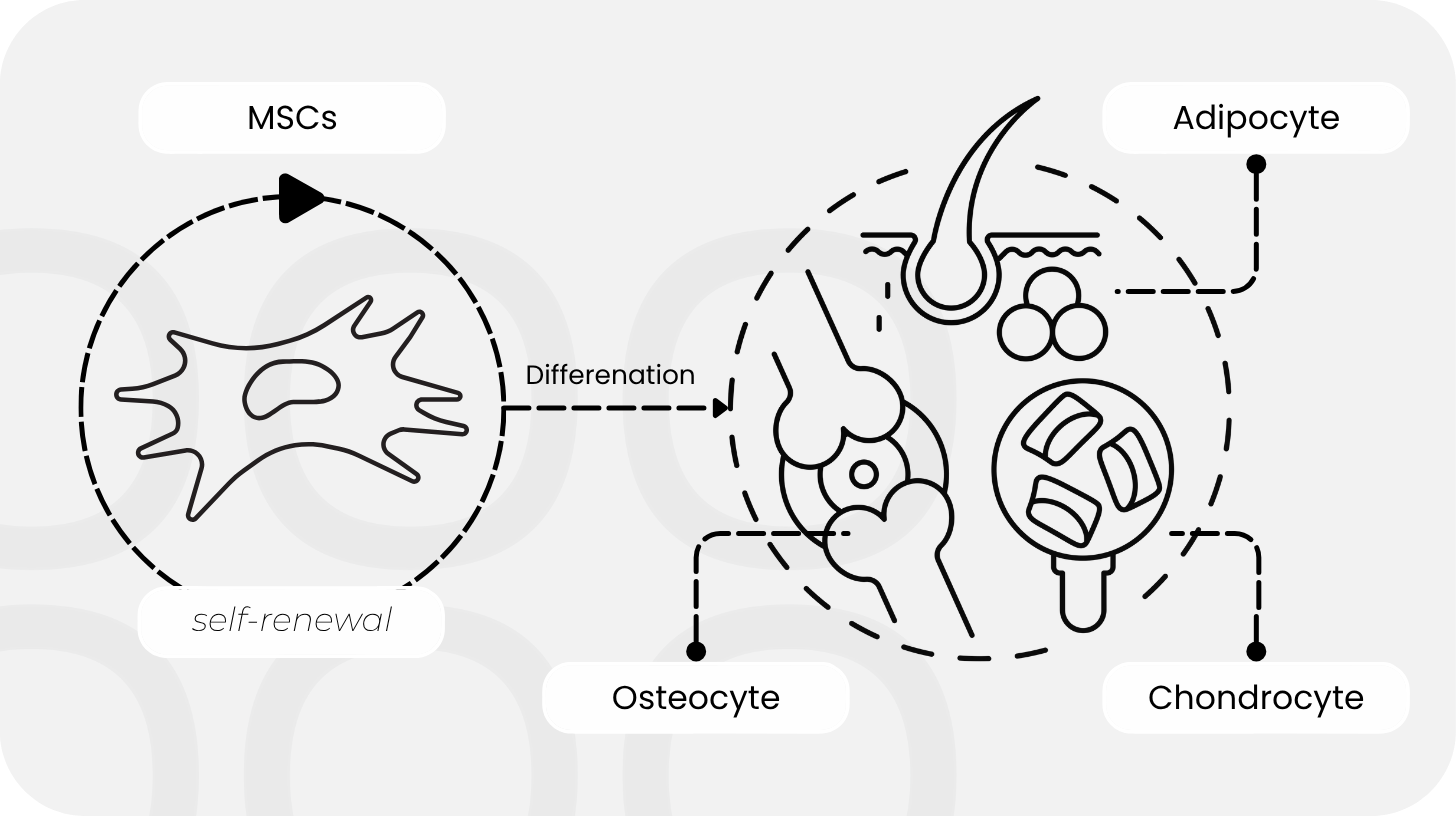
Yet their true strength lies not in their ability to transform, but in what they secrete. MSCs communicate with their environment through signals such as cytokines, growth factors, and, above all, tiny packaged particles known as exosomal vesicles. They modulate inflammation, promote healing, and regulate immune responses. Researchers refer to this as the paracrine effect: MSCs act as biological communication hubs that instruct other cells to self-regulate or even regenerate.
Recent international studies have shown that mesenchymal stem cells can regenerate damaged cells via their paracrine effect by transferring functional mitochondria through vesicles, a mechanism gaining attention in neuro- and cardiac research for its significant therapeutic potential.² This finding fundamentally expands our understanding of “cell therapy”: it’s not only the cells themselves, but also their signals, that can serve as therapy.
With new technologies like CRISPR-Cas9 and RNA editing, MSCs are now being precisely modified to become more robust, release specific molecules, or migrate more accurately to injured tissue.³ Researchers from South Korea and the U.S. demonstrated in 2020 that MSCs and their exosomes can sense local cytokine environments and actively modulate inflammatory processes. These natural self-regulation mechanisms enable MSC-derived exosomes to dampen inflammation, promote wound healing, and support skin regeneration.⁴
Some teams are even combining gene editing with synthetic biology to turn cells into programmable therapeutic carriers. By merging gene editing, induced pluripotent stem cells, and MSCs, scientists are creating powerful tools, so-called GE-iPS-MSCs, that not only heal but also perform monitoring functions: they can be programmed to detect stress, pH shifts, or oxygen deficiency and respond by releasing cytokines or growth factors. The result marks a shift from cell transplantation to intelligent cell technology, a fusion of biology and machine logic.⁵
While research continues to refine MSCs, another trend is emerging: the step away from the cell itself. Exosomes, the small vesicles naturally released by MSCs, are increasingly regarded as standalone therapeutics. They carry microRNAs, proteins, and lipids that orchestrate cellular communication, and they are more stable, easier to store, and simpler to regulate than whole cells.⁵ These “cell-free MSC therapies” may bridge the gap between research and clinical practice: while living cell products still require individualized logistics, exosomes are, in theory, as storable as biopharmaceuticals.
One of the most promising frontiers for mesenchymal stem cells lies in diabetes research. Here, MSCs are not only explored as regenerative agents but also as powerful immunomodulators. Clinical and preclinical studies suggest that they can help preserve pancreatic β-cell function, calm autoimmune activity in type 1 diabetes, and improve insulin sensitivity in type 2 diabetes. At the same time, researchers are developing exosome-based therapies that may deliver similar effects.⁶ While the road to clinical routine remains long, the potential impact of MSC-based and exosome-driven therapies on metabolic diseases is enormous.
But the race remains open: which technology will prevail depends on how well efficacy, safety, and manufacturing costs can be balanced. While exosomes redefine the biochemical communication networks of MSCs, advances in 3D bioprinting are reshaping their structural and mechanical environment.
New Dimensions in Tissue Engineering
A new wave of innovation arises from the combination of mesenchymal stromal cells with 3D bioprinting and advanced biomaterial scaffolds. Instead of growing in flat cultures, MSCs are now cultivated in three-dimensional structures that mimic their natural environment, guiding differentiation, survival, and regeneration. Researchers worldwide are developing conductive and stimuli-responsive hydrogels that respond to mechanical or electrical cues to regulate cell activity. In parallel, multi-phasic bioprints are being explored, constructs with varying material properties capable of regenerating bone and cartilage simultaneously.⁷ These advances show that regeneration depends not only on the cell itself but also on its microenvironment. Factors like oxygen, temperature, shear forces, and signaling molecules determine how tissue forms and heals. Understanding these parameters is key to translating 3D-printed MSC constructs safely and reproducibly into clinical application.
The greatest hurdle, however, remains large-scale manufacturing: some MSC therapies require hundreds of millions of cells, and each passage or media change can subtly alter their properties. This makes standardization and regulatory control highly demanding. Unsurprisingly, biotech companies worldwide are investing in automated culture systems and closed bioreactors to produce reproducible cell batches.
At the same time, regulatory pressure is increasing: in the United States, the Food and Drug Administration (FDA) released several draft guidance documents in 2024 for cell and gene therapy products, outlining stricter criteria for characterization, process control, and quality assessment of so-called Cell and Gene Therapy Products (CGTs). The goal is to improve comparability and safety while providing manufacturers with clearer validation and documentation requirements. European and Asian regulators, including the European Medicines Agency (EMA) and the Health Sciences Authority (HSA) in Singapore, are working in parallel on similar frameworks to harmonize global standards for cell-based therapies and accelerate product approvals.
Yet all quality guidelines mean little if the greatest unknown lies outside the laboratory. In clinical reality, MSCs are rarely used where they are produced, they often travel across the country or continents. And while they travel, they remain alive. That may sound trivial, but it’s crucial: every hour outside optimal conditions alters their vitality and potency. Temperature, gas, and pressure fluctuations during transport can irreversibly change cellular physiology. Some clinical studies underestimate how strongly such logistical factors affect therapeutic success. According to a 2024 publication in the Journal of Translational Medicine, the number of treatable patients is limited in part by logistical challenges in manufacturing, transport, and application of these products.⁸
This makes it clear: the success of an MSC therapy depends not only on which cells are used, how they are prepared, and how they are administered, but also on how they survive the journey from production sites to patients.
Perhaps this is the most subtle, yet most important, lesson of MSC research: life cannot be frozen without being changed. Many of today’s cryopreservation methods date back to a time when they were developed for stable cell lines, not for sensitive, differentiated MSCs, simply because there were no alternatives. Studies now show that even short periods of deep cooling can impair cell membranes, mitochondrial structures, and intracellular signaling pathways, reducing long-term viability.⁹ While 37 °C and 5 % CO₂ are the undisputed standard for cell growth under controlled culture conditions, these physiological parameters are rarely considered in the context of transport and interim storage, even though compromises and cellular alterations associated with cryopreservation have long been recognized.
This is precisely where technology companies like Cellbox Solutions come in: developing transport systems that allow cells to travel around the world in mobile incubators without losing their biological balance.
When we summarize the developments of recent years, a clear picture emerges:
→ MSCs are no longer a niche phenomenon but a cornerstone of regenerative medicine.
→ Their applications range from anti-inflammatory therapies to tissue and organ regeneration.
→ New technologies (from exosomes to 3D bioprinting) continue to expand their potential.
→ Yet they unfold their full impact only when we recognize that cell biology does not end at the Petri dish, but continues with every movement, every temperature change, and every hour in transit.
The future of MSCs will be interdisciplinary. Biologists, engineers, material scientists, logisticians, and clinicians will work together to move living systems safely. The story of MSCs teaches us that progress does not lie solely in discovery but in understanding the connections: between cell and environment, research and application, science and logistics.

