June 2025

Hydrogels in Advanced Therapies: Pioneering a New Era in Regenerative Medicine
Written by: Robin Sieg & Prof. Dr. Kathrin Adlkofer
The promise of regenerative medicine has never been greater. With the global market projected to exceed USD 154 billion by 2033¹, the field is no longer a distant hope but a rapidly unfolding reality. Scientific breakthroughs are transforming what once seemed impossible—offering the prospect of repairing damaged hearts, restoring lost function after injury, or even replacing failing organs with living tissue. For patients facing life-limiting conditions, these advances represent far more than innovation; they embody a renewed chance at life. Yet as medicine moves closer to making regeneration routine, new questions emerge. How can we ensure that their potential translates into consistent, reliable outcomes? Among the emerging answers are advanced biomaterials—particularly hydrogels—that are quietly reshaping the future of regenerative therapies by addressing some of the field’s most complex challenges.
Hydrogels are soft, three-dimensional polymer networks that retain large volumes of water and mimic the properties of living tissue². Their biocompatibility and permeability make them ideal for supporting embedded cells. Nutrients, oxygen, and signaling molecules can freely diffuse through the gel, while the cells remain cushioned within a biomimetic scaffold. But hydrogels are more than passive carriers. They are dynamic platforms engineered to protect, nurture, and even direct the behavior of therapeutic cells. Their softness belies their impact. Today, hydrogels are increasingly being integrated into cell therapies not only to support viability, but also to tackle one of regenerative medicine’s most persistent challenges: the body’s immune response.
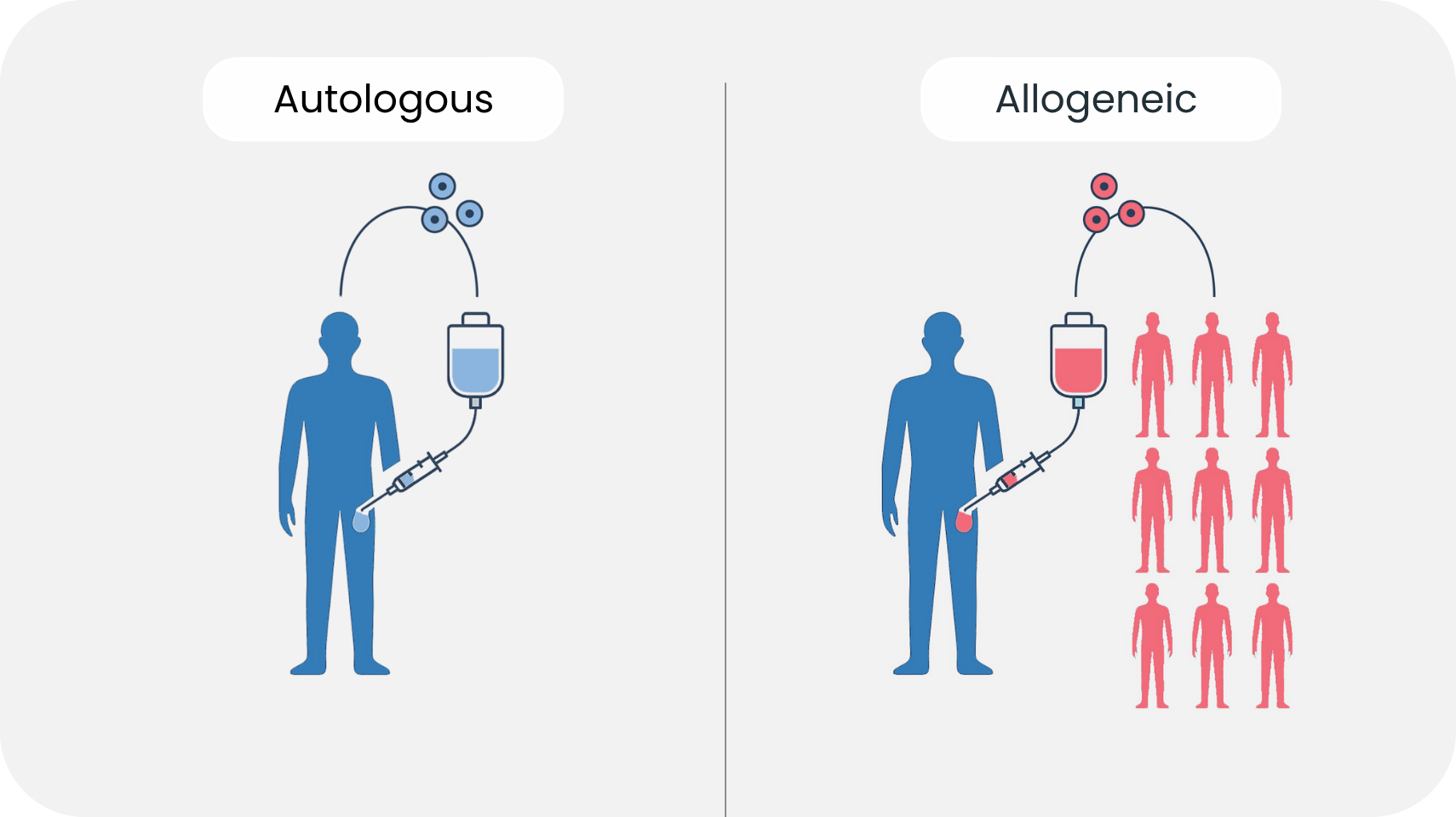
Autologous therapies—where a patient's own cells are harvested, modified, and reintroduced—remain the current standard in many applications, especially in oncology and hematology³. However, autologous approaches are often expensive, time-intensive, and dependent on the patient’s health status. Allogeneic therapies, where donor cells are used, offer scalability and off-the-shelf availability, but they come with a significant drawback: the recipient's immune system often perceives these cells as foreign and mounts an attack⁴. Immune rejection can destroy the therapeutic cells before they ever have a chance to act. In response, scientists have long sought ways to cloak donor cells from immune detection.
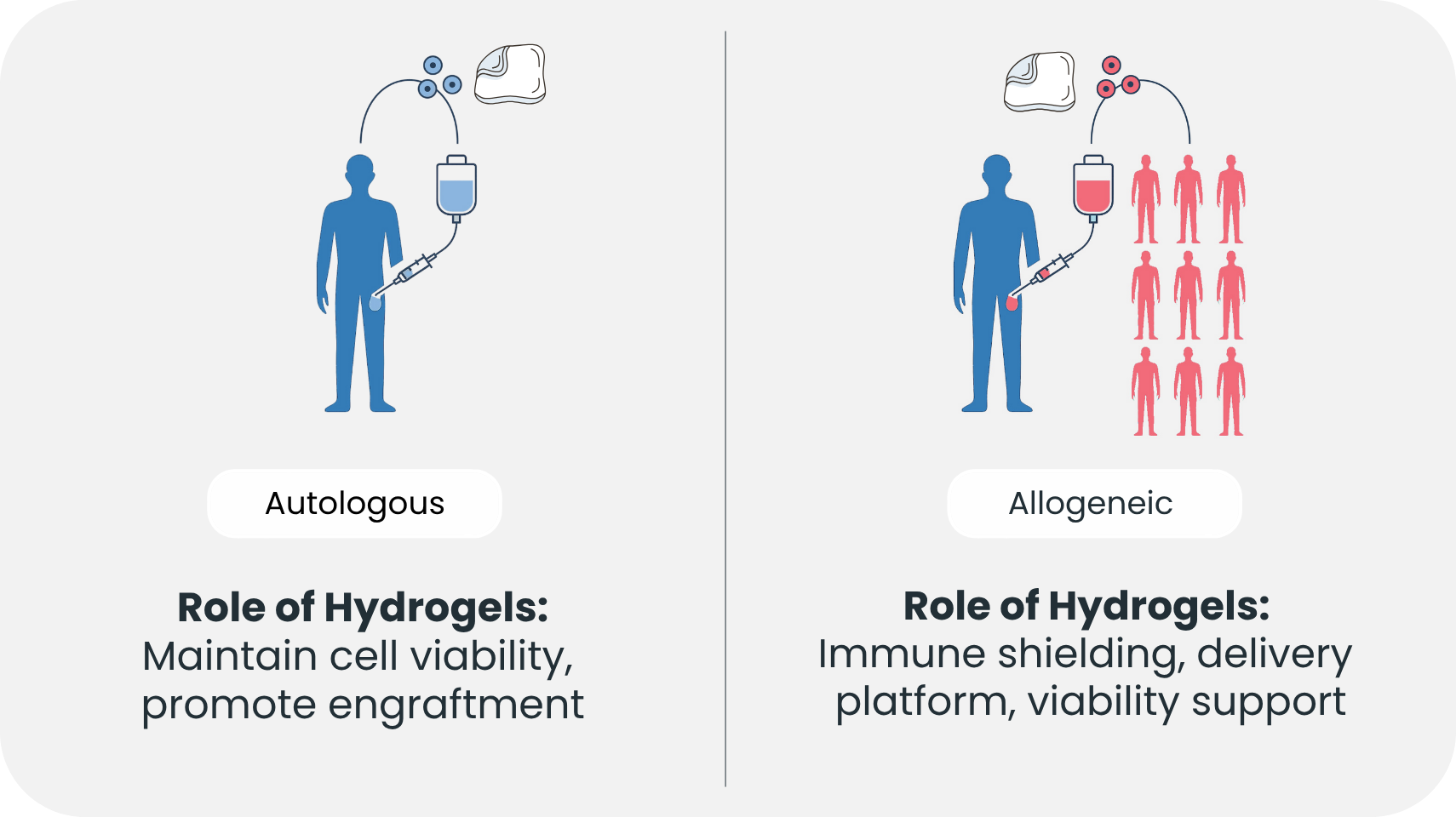
Hydrogels offer a novel solution. By encapsulating cells within a semi-permeable hydrogel, direct physical contact with host immune cells can be avoided. This immune isolation allows the embedded cells to release therapeutic molecules such as hormones, enzymes, or cytokines while remaining undetected by cytotoxic immune cells⁴. In essence, hydrogels act as a molecular sieve—permitting life-sustaining interaction while shielding the graft from immunological attack.
One remarkable demonstration of this approach involves islet cell therapy for Type 1 diabetes. Encapsulation of insulin-producing beta cells in alginate-based hydrogels has shown in preclinical models that blood sugar levels can be regulated without systemic immunosuppression⁴. This is no minor feat. Immunosuppressive drugs, while effective in dampening immune responses, expose patients to infections and malignancies. A hydrogel-based therapy that avoids this trade-off could be transformative.
But passive protection is just the beginning. Researchers are now engineering hydrogels with active immunomodulatory properties. These advanced biomaterials are infused with nanoparticles, extracellular vesicles, or immune-regulating ligands that can modulate the local immune environment. A recent study featured a hydrogel embedded with vesicles from mesenchymal stem cells carrying FasL and PD-L1 proteins—both known to inhibit T cell activation. When used in transplant models, the hydrogel not only prevented rejection but also promoted the expansion of regulatory T cells, which are essential for long-term immune tolerance⁴.
Such innovations elevate hydrogels from inert scaffolds to immune-savvy gatekeepers. By fine-tuning the cellular milieu, they help foster an environment conducive to tissue integration and long-term function. For patients and clinicians, this could mean fewer immune-related complications and more durable therapeutic outcomes.
In the clinical realm, hydrogels are already transforming practice. In orthopedics, injectable hydrogel matrices seeded with stem cells have been used to regenerate cartilage in osteoarthritic joints, demonstrating lasting improvement in mobility and pain reduction³. In wound healing, hydrogel-based dressings are routinely employed to accelerate re-epithelialization and reduce scarring, especially in burn and diabetic foot ulcers². In cardiovascular research, preclinical trials are exploring hydrogel patches embedded with cardiomyocytes to repair infarcted myocardium. These hydrogels not only serve as structural scaffolds but may also release pro-angiogenic factors, aiding vascular regeneration. The convergence of material science and biology is yielding tools that restore function in ways traditional medicine cannot.
However, even the most advanced therapeutic materials are only as effective as the logistics that deliver them. Transporting living cells remains a daunting challenge. Cryopreservation—the old standard —requires ultra-low temperatures to pause cellular metabolism, often at -80°C or in liquid nitrogen⁵. Although effective for long-term storage, the freeze-thaw cycle damages cell membranes, disrupts protein structures, and significantly reduces viability. Cryoprotectants like DMSO, used to mitigate this damage, introduce toxicity risks of their own⁶. The result is a compromise: we freeze cells to preserve them, yet we lose a portion—and sometimes their functionality—in the process.
Hydrogels offer a compelling alternative in this regard. Studies show that certain hydrogel formulations can maintain high levels of cell viability at room or physiological temperature for several days⁷. For example, mesenchymal stem cells encapsulated in alginate or hyaluronic acid gels demonstrated sustained viability and functional properties even after extended ambient transport. This eliminates the need for complex cold-chain infrastructure and reduces turnaround time for clinical application.
To support this logistical shift, devices like the Cellbox portable incubator have emerged. Designed to maintain controlled temperature and CO₂ levels, Cellbox enables the transport of live-cell preparations under physiological conditions. When used in conjunction with hydrogel-based cell therapies, such technologies offer a powerful synergy: biological stability meets environmental control. Cells can be shipped viable, active, and ready for immediate clinical use—without thawing, recovery, or risk of loss.
Meanwhile, hydrogel research continues to push boundaries. New formulations are stimuli-responsive, releasing their payload in response to specific biological cues such as inflammation or changes in pH². Magnetically or light-triggered hydrogels allow clinicians to control therapeutic delivery noninvasively, introducing the concept of remote-regulated cell therapies. Other efforts focus on accelerating the bioprocessing phase. One study demonstrated that a 3D zwitterionic hydrogel platform increased hematopoietic stem cell yield by 73-fold, offering an efficient alternative to traditional culture systems². For CAR-T or NK cell therapies, such innovations could dramatically shorten production timelines.
The implications are far-reaching. For clinicians, hydrogel-based systems offer tailored solutions adaptable to a wide range of therapeutic challenges. For investors, they represent a convergence of biomaterials and biotech with high translational value. For patients, they offer a pathway to treatments that are not only effective but accessible, timely, and safer than ever before.
As we envision the next decade of regenerative medicine, one thing becomes clear: hydrogels will no longer be peripheral. They are becoming central pillars of how we deliver, protect, and enhance living therapies. They are the scaffolds that preserve viability, the shields that guard against rejection, and the vehicles that ensure that no cell, and no patient, is left behind.

