January 2025
Cell and Gene Therapy: The Last Hope for Patients with Deadly Diseases – Why the Quality of Biological Products Is So Crucial
Written by: Robin Sieg & Prof. Dr. Kathrin Adlkofer
Every year, approximately 20 million people are diagnosed with cancer worldwide1. In 2022 alone 9.7 million people lost their fight against this devastating disease¹. Adding to this, around 53.5 million people worldwide are currently living with or recovering after a cancer diagnosis². The burden of this disease extends further: A study from Norway found that approximately 3.1% of minors and 8.4% of young adults have a parent who has been diagnosed with cancer³. Millions of others live in the shadow of uncertainty, clinging to the hope for a therapy that often arrives too late. For many of these patients, cell and gene therapies are the last chance to turn a hopeless diagnosis into a cure. However, as revolutionary as these approaches are, they face formidable challenges that significantly limit their dissemination and effectiveness.
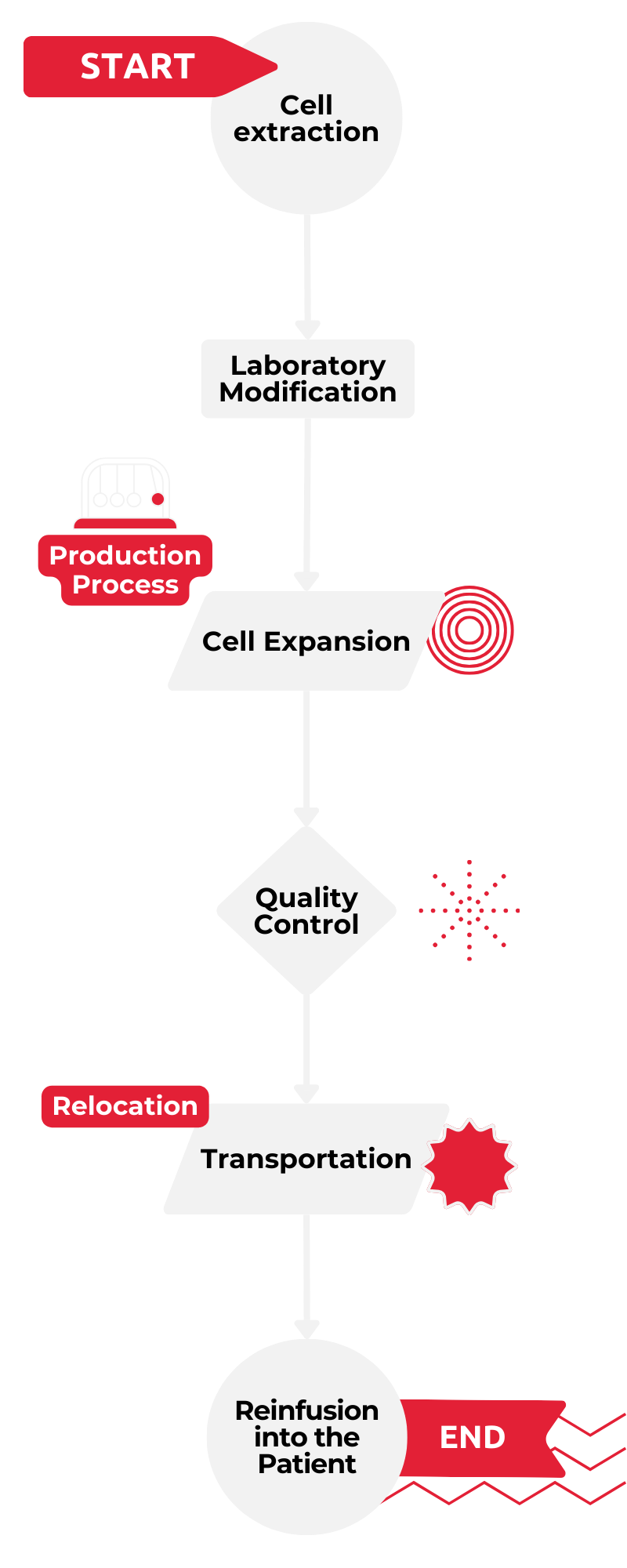
Cell and gene therapies provide a promising approach to treating serious conditions such as cancer, autoimmune diseases, and genetic disorders and often represent the last resort for patients to survive. Among other cell therapies, such as CAR-T cell therapy, NK (natural killer) cell therapies are particularly noteworthy for their ability to utilize immune cells to identify and destroy malignant cells. This process begins with isolating NK cells from the patients themselves, followed by precise laboratory modifications and their subsequent multiplication. Once reintroduced to the patient, these modified cells can unfold their highly specific therapeutic effects. This method has already demonstrated impressive outcomes, including sustained remissions of late stage cancers.
However, this revolutionary technology is not without its challenges. The production process is not only complex but also time-consuming and expensive. Each therapy is tailored to the patient, requiring highly skilled personnel and specialized laboratories. Moreover, logistical challenges arise as production often takes place in centralized facilities, necessitating time-sensitive transportation, which is both costly and prone to risks, such as compromising quality or even loss of the therapeutic cells.
A key issue is the quality of the cells used. Many patients are already severely weakened by chemotherapy or radiotherapy and have only limited quantities of healthy cells available. To achieve optimal results, the cell products must be of the highest quality, with maximum viability and functionality. In the patient's body, the cells must be able to implant themselves successfully, multiply, and exert their therapeutic effect. Even the slightest compromise in cell quality can endanger the therapy's success.
A crucial aspect of cell therapy is the development of new technologies that can improve the quality and efficiency of production. Standardization of processes plays a central role here. Automated systems for cell culture and manipulation could help to reduce sources of error and increase the consistency of results. In addition, advanced analysis tools can be used to monitor the properties of the cells during production. This allows potential problems to be identified and resolved at an early stage.
Costs and the time required represent another hurdle. The entire process, from cell removal to modification and reinfusion, can take weeks—time that many patients often do not have. At the same time, the cost per therapy can run into the hundreds of thousands of euros, which significantly limits access. Innovative approaches to cost reduction are needed, for example, by developing scalable production processes or using new materials that make cell culture more efficient.
A particularly exciting area of research is allogeneic therapies, which use healthy donor cells rather than the patient’s own cells. These approaches could circumvent the need for individualized manufacturing and reliance on the patient’s own cells with sometimes limited usability, thus significantly reducing production time and costs. However, allogeneic cell therapies often face the challenge that one needs to mitigate detrimental immune reactions of the patient’s immune system against the cells and thus ensuring the long-term efficacy of the cells. Research in this area could fundamentally change the field of cell therapy and significantly expand access to these innovative treatments.
Despite these challenges, cell therapy offers an enormous opportunity to fundamentally change the way we bring medical treatment to the patients. It has the potential to give a second chance to patients who were previously considered untreatable. Multicenter studies could be facilitated by advances in logistics, allowing a larger patient population to be reached. These therapies could not only save lives but also significantly improve the quality of life of patients with autoimmune diseases and genetic disorders.
Flow Chart: Cell and Gene Therapy Treatments
This is where Cellbox Solutions' mission comes in. We strive to significantly improve the logistics processes of cell therapy and to maintain the maximum potency of the cell products. Our research indicates that innovative solutions can increase both the efficiency and the effectiveness of these therapies. By working together with research institutions and clinical centers at an early stage, we want to ensure that logistical challenges are addressed from the onset. Our vision is not only to prolong the lives of patients worldwide, but also to help make them worth living again
One of our central innovations focuses on advancing transport technologies that ensure cells remain in optimal condition throughout transit between the clinics and specialized manufacturing centers. Our goal is to transport cells over longer distances without compromising viability or functionality. However it is not only to facilitate logistical planning and mitigate risks in shipping valuable goods but also to support multicenter studies and thereby accelerate clinical research.
Portable Incubators With Active CO₂ And Temperature Control.

Logistical Challenges and Opportunities
The logistics of cell therapy is often complex and requires a thorough understanding of the requirements of living cell products. A primary challenge lies in maintaining a stable and controlled environment for the cells during transport. Temperature and CO₂ levels must be closely controlled to ensure that the cells retain their functionality. Here, innovative transport solutions equipped with state-of-the-art sensors and real-time monitoring can make a big difference.
In addition, international logistics require close coordination between manufacturers, logistics service providers, and clinical centers. Differences in regulatory requirements and customs regulations can further complicate the process.
Developing standardized logistics procedures within a network of regional production sites could help overcome these challenges and reduce dependence on centralized locations.
Research into innovative solutions in logistics offers a tremendous opportunity to increase the efficiency of cell therapy and reduce its costs. At Cellbox Solutions, we have developed technologies such as the Cellbox™ Shipper and Cellbox Go to address these challenges. The Cellbox™ Shipper is designed for long-distance transport, maintaining optimal conditions for living cell products by ensuring precise control of the incubation temperature and CO₂ concentration. It integrates advanced monitoring systems to maintain cell viability throughout the journey. Moreover, the Cellbox™ Go provides a compact, portable solution for short transport needs, ideal for clinical settings requiring rapid and reliable transfers. By leveraging these innovations, we are helping to streamline logistical operations, reduce risks, and ensure that high-quality cell therapies reach patients wherever and whenever they are needed. Together, these solutions make a significant contribution to overcoming the imminent logistical barriers in cell therapy.
Cell therapy is much more than a medical innovation. It is a beacon of hope for millions of patients worldwide. To realize its full potential, we must overcome the existing hurdles: from ensuring cell quality and efficient logistics to reducing costs and time.
Imagine a world in which every patient, regardless of location or financial situation, has access to proven life-saving cell therapies or can benefit from participation in a clinical trial. This vision is within reach, and at Cellbox Solutions, we are actively seeking partners to test and integrate our innovative products in cell therapeutic applications. Together, we can advance this medical revolution, improve access to these life-changing treatments, and shape the future of medicine.
We invite you to collaborate with us to advance this medical revolution and improve access to life-changing treatments.
Sources
³ https://pubmed.ncbi.nlm.nih.gov/22442635/

